11 de November de 2020
Sport tech and science Strength and science
Unique properties and adaptations followed by eccentric training
Everyone in the sports science world has ever heard of the pioneering work of Archibald V. Hill in 19221 and 19382 about the relationships among muscle force, velocity and power. His work not only derived in the award-winning of the 1922 Nobel Prize in Medicine but also it was a cornerstone of muscle physiology field with ramifications and implications in multiple aspects of sports sciences.
For decades, textbooks classified muscle use during movement and activity as situating into one of these categories, isometric or isotonic. Both forms of muscle contraction fit perfectly with the dominant theory of the molecular mechanisms of muscle contraction called the sliding filament theory3.
However, when the magnitude of a force applied to a muscle exceeds that produced by the muscle, the muscle will lengthen as work is done on it (often called “negative work”). This lengthening while loading muscle contraction is known as eccentric muscle contraction and the term was first introduced by Asmussen as “eccentric” which comes from ex-, “from or away,” and centric, “centre,” hence it´s implied that the muscle slides “away” from its middle point4.
This type of muscle contraction actually, force scientists to redefine muscle contraction. The dictionary definition of the verb “to contract” specifically for the case of muscle, is “to undergo an increase in tension, or force, and become shorter.” Under all circumstances, an activated muscle generates force, but an activated muscle generating force does not invariably shorten as seen during an eccentric contraction5. Depending on the interaction between the force developed by the muscle and the load on the muscle, the muscle will either shorten, remain at a fixed length (isometric), or be lengthened. The recognition that muscles perform three different types of “contractions” required that contraction be redefined as “to undergo activation and generate force”5.
According to Faulkner to clarify the type of contraction, the adjectives that provide the greatest clarity are “shortening,” “isometric,” and “lengthening”5. However, even that my personal opinion is in line with the aforementioned author, to make this blog post easier to understand I’m going to interchangeably use the terms “eccentric” and “lengthening under load”.
The value of eccentric exercise has often been questioned because of the fact that eccentric contractions in people unaccustomed to this type of exercise can lead to muscle soreness and muscle damage. However, eccentric muscle contractions are important in practically all sports that involve jumps, running, or throwing as a critical part of the stretch-shortening cycle6
Cross Bridge Cycle and Sliding Filament Theory
During muscle contraction, the filaments actin and myosin remain at a constant length and a change in fibre length is achieved via a change in the overlap between the two in a sliding motion; hence the sliding filament theory of muscle contraction7,8.
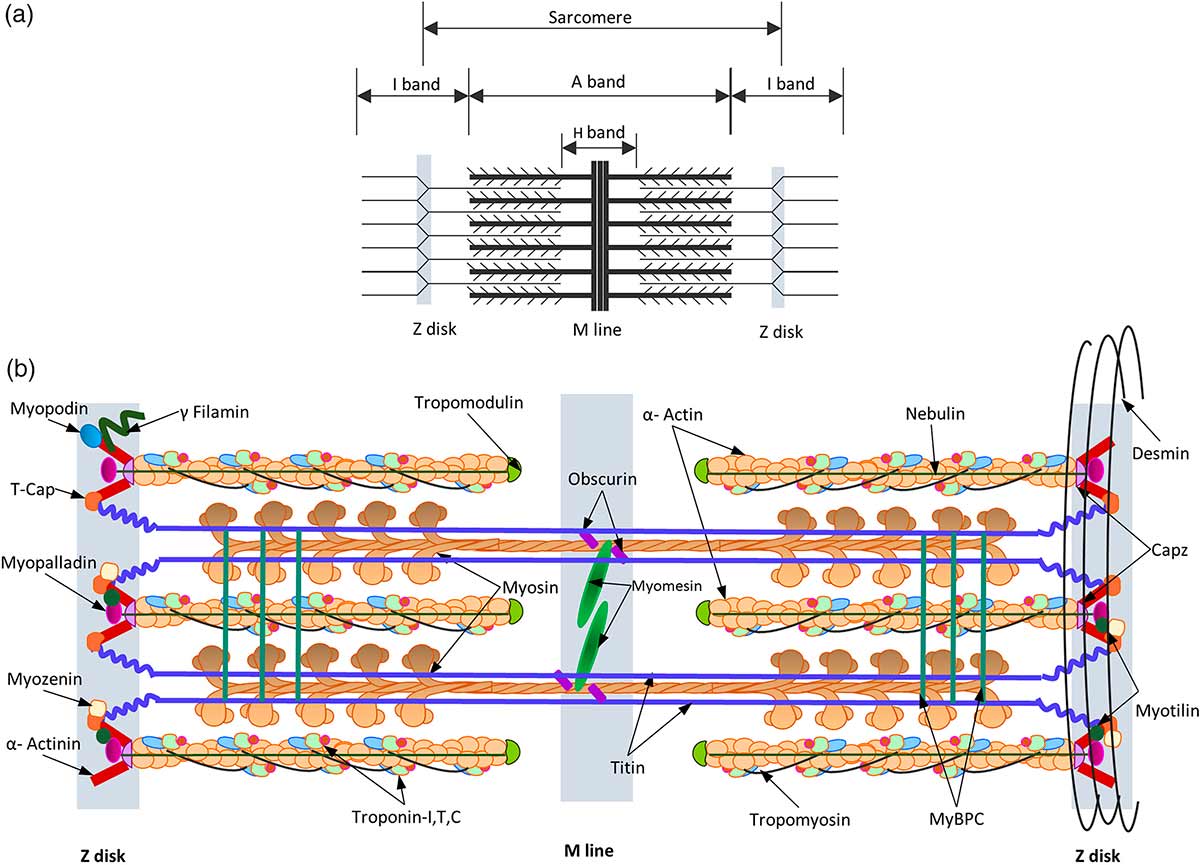
Figure 1 Schematic representation of the sarcomere. The thick filaments, thin filaments, Z- and M-lines are indicated by blue, red, yellow and green boxes, respectively. The protein components shown to localise in the sarcomere have been directly labelled on the appropriate area of the ultrastructure9
The myosin cross-bridges generates the driving force for the sliding motion. Myosin heads interact with actin through binding sites repeatedly and as the number of cross-bridges formed increases, the magnitude of contractile activation and amount of actin-myosin overlap growth (i.e. the length-tension relationship). 10.
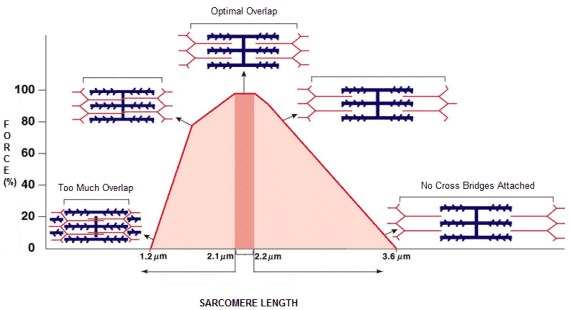
Figure 2 – This theoretical process explains both concentric and isometric contraction10.
In the case of isometric actions, where there is no change in muscle length, actin-myosin overlap does still occur with bridges spontaneously dissociating and being replaced by new ones 11. When the external load is overcome by the applied force, the muscle shortens and as speed increases, the time myosin heads is exposed to actin-binding sites decreases and the number of cross-bridges that may be formed is less (i.e. a force-velocity relationship). However the cross- bridge theory alone is not sufficient to explain neither the greater force produced during active lengthening nor the time-dependent residual force enhancement (this is mainly seen during the Strength shortening cycle) 12,13.
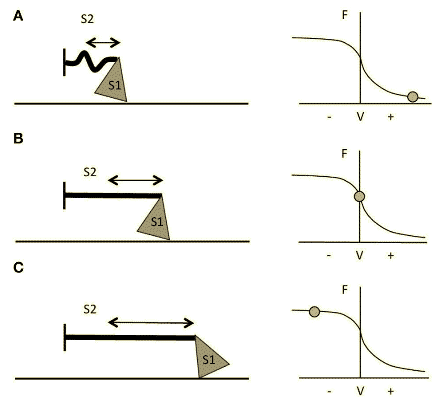
Figure 3 Schematic representation of myosin S1 and S2 segments behavior during different contractions and at different respective portions of the F-V curve. (A) Fast Strength-shortening cycle(SSC) actions, S2 will not fully stretch and myosin will apply less force through actin. (B) During slower shortening contractions, up to when the shortening velocity would be equal to 0 (i.e., isometric contractions), the S2 segment will be fully stretched and therefore myosin will be able to apply greater force. (C) During lengthening actions, myosin S2 complex will be able to stretch even further14.
Several theories have been proposed to understand those gaps regarding eccentric contraction characteristics that cannot be explained by sliding filament theory. The increased force production during lengthening above isometric force ranges may be related to differences in the number of attached cross-bridges and mechanical detachment of active cross-bridges. It has been proposed that the second head of a myosin molecule can attach to actin facilitated by the increased strain on a single myosin head during lengthening contractions12. Furthermore, it seems that cross-bridges do not complete a full cycle during eccentric contractions15; they become suspended in an active state bound to actin and become forcibly detached followed by a rapid re-attachment16.
More intriguingly, the enhancement of force produced followed by an eccentric action indicates that passive elements beyond cross-bridge mechanisms may be involved17. Even though is yet not fully understood, it seems that this passive component is related to changes in a protein called Titin which has been shown to have spring-related properties and is involved in the stiffness of the sarcomere17–19.
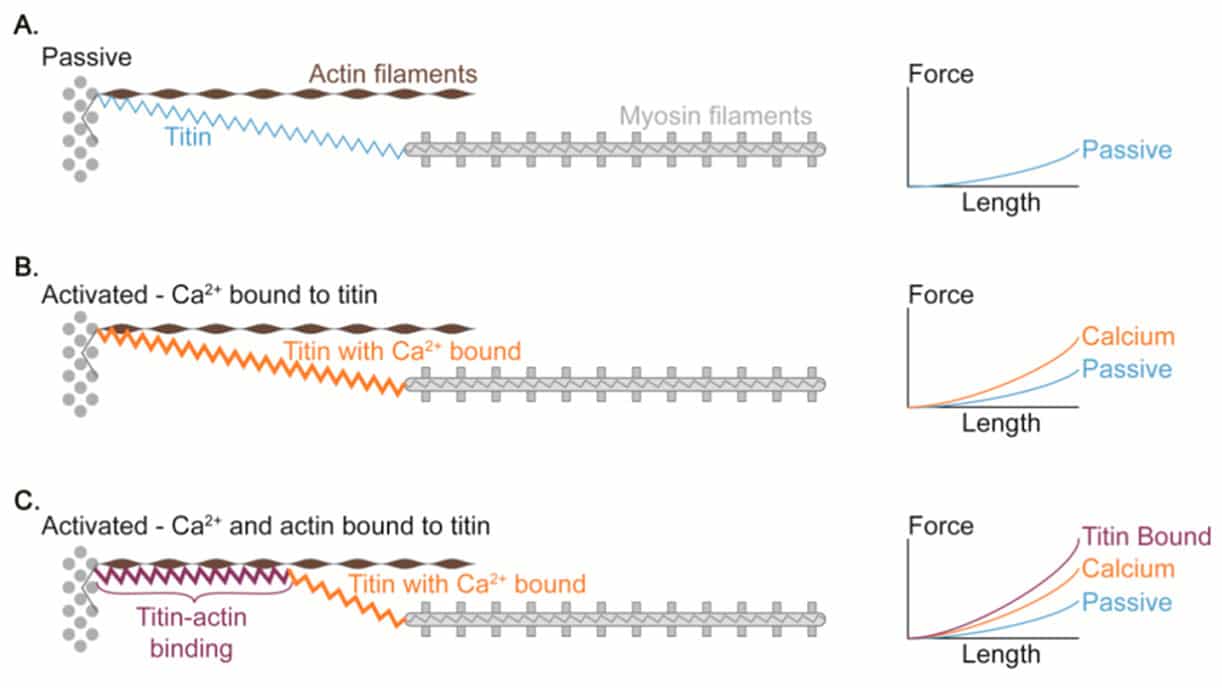
Figure 4 Proposed mechanism of titin-actin interaction.
Titin is the largest protein currently known in the natural world 21, and is an important structural component of the muscle cytoskeleton. Because of its location in the sarcomere, titin produces elastic force when the sarcomere is elongated. Several models of how titin works have been proposed such as the “winding filament” hypothesis22, Titin-actin interactions and titin-myosin interactions23.
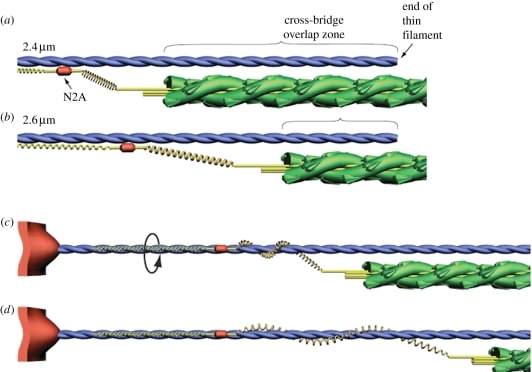 Figure 5 Schematic illustrating the winding filament hypothesis22
Figure 5 Schematic illustrating the winding filament hypothesis22
Eccentric contraction-induced adaptations.
The uniques morphological and neural characteristics of the eccentric actions, transform this type of contraction in a powerful tool for not only maximizing performance but also as both rehab and prehab effective strategy. During the last decades, it has gained a growing interest in several fields beyond sports training or physical medicine and rehabilitation. Evidence is accumulating regarding the benefits of eccentric exercise in special populations of aged individuals or patients with chronic health diseases such as neuromuscular pathologies24–26.
Structural Adaptations
Hypertrophy is the result of an increased protein translation, upregulation of genes involved in anabolic mechanisms, and satellite cell activation/proliferation27. Muscles have the ability to convert mechanical signalling to a molecular one wihch involves the upregulation of primary and secondary messengers within a signalling cascade to activate and/or repress pathways that regulate gene expression and protein synthesis/degradation28.
The main factors are believed to be responsible for the hypertrophic signalling response to training; mechanical tension, muscle damage, and metabolic stress29. However, recent data indicates that both muscle damage and metabolic stress alone are not sufficient to promote muscle hypertrophy, they could have an amplifier role when mechanical tension reaches a certain threshold30,31
In this scenario, the potential role of eccentric contraction to promote muscle growth may be due to higher levels of both mechanical tension and muscle damage than concentric training32. High levels of tension induce a mechanochemical signal to upregulate anabolic molecular and cellular activity within myofibers and satellite cells; the combined effects of active tension from contractile elements and passive tension from collagen content within the extracellular matrix and titin are believed to induce a more potent signal for protein synthesis33. However, when eccentric training modalities are compared with traditional resistance training there are no significant differences in measures of hypertrophy27,34.
Contraction type appears to mediate a region-specific hypertrophy; eccentric training tends to induce greater increases in distal muscle size, while mid-muscle hypertrophy occurs to a greater extent following concentric training35,36. Additionally, fibre type composition may be uniquely influenced by eccentric training, and either increase or maintenance, of IIx(IIb) fibres, has been found compared with concentric training27,36,37.
You can continue reading the second part of this article here.
If you have any questions, don’t hesitate to ask! Leave a comment or contact us here.
Bibliography
1.Hill, A. V. The maximum work and mechanical efficiency of human muscles, and their most economical speed. The Journal of Physiology 56, 19–41 (1922).
2.The heat of shortening and the dynamic constants of muscle. Proceedings of the Royal Society of London. Series B – Biological Sciences 126, 136–195 (1938).
3.Cooke, R. The Sliding Filament Model. Journal of General Physiology 123, 643–656 (2004).
4.ASMUSSEN., E. Positive and Negative Muscular Work. Acta Physiologica Scandinavica 28, 364–382 (1953).
5.Faulkner, J. A. Terminology for contractions of muscles during shortening, while isometric, and during lengthening. Journal of Applied Physiology 95, 455–459 (2003).
6.Hoppeler, H. & Herzog, W. Eccentric Exercise: Many questions unanswered. Journal of Applied Physiology 116, 1405–1406 (2014).
7.HUXLEY, A. F. & NIEDERGERKE, R. Structural Changes in Muscle During Contraction: Interference Microscopy of Living Muscle Fibres. Nature 173, 971–973 (1954).
8.HUXLEY, H. & HANSON, J. Changes in the Cross-Striations of Muscle during Contraction and Stretch and their Structural Interpretation. Nature 173, 973–976 (1954).
9.Mukund, K. & Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdisciplinary Reviews: Systems Biology and Medicine (2019) doi:10.1002/wsbm.1462.
10.Douglas, J., Pearson, S., Ross, A. & McGuigan, M. Eccentric Exercise: Physiological Characteristics and Acute Responses. Sports Medicine 47, 663–675 (2016).
11.Herzog, W., Powers, K., Johnston, K. & Duvall, M. A new paradigm for muscle contraction. Frontiers in Physiology 6, (2015).
12.Linari, M. et al. A combined mechanical and X-ray diffraction study of stretch potentiation in single frog muscle fibres. The Journal of Physiology 526, 589–596 (2000).
13.Edman, K. A., Elzinga, G. & Noble, M. I. Residual force enhancement after stretch of contracting frog single muscle fibers. The Journal of General Physiology 80, 769–784 (1982).
14.Franchi, M. V., Reeves, N. D. & Narici, M. V. Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Frontiers in Physiology 8, (2017).
15.Linari, M. et al. The mechanism of the force response to stretch in human skinned muscle fibres with different myosin isoforms. The Journal of Physiology 554, 335–352 (2004).
16.Huxley, A. F. Biological motors: Energy storage in myosin molecules. Current Biology 8, R485–R488 (1998).
17.Herzog, W. The role of titin in eccentric muscle contraction. Journal of Experimental Biology 217, 2825–2833 (2014).
18.Herzog, W., Leonard, T. R., Joumaa, V. & Mehta, A. Mysteries of Muscle Contraction. Journal of Applied Biomechanics 24, 1–13 (2008).
19.Morikawa, S., Inubushi, T., Kito, K. & Tabata, R. Imaging of phosphoenergetic state and intracellular pH in human calf muscles after exercise by 31P NMR spectroscopy. Magnetic Resonance Imaging 12, 1121–1126 (1994).
20.Fukutani, A. & Herzog, W. Current Understanding of Residual Force Enhancement: Cross-Bridge Component and Non-Cross-Bridge Component. International Journal of Molecular Sciences 20, 5479 (2019).
21.Leonard, T. R. & Herzog, W. Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. American Journal of Physiology-Cell Physiology 299, C14–C20 (2010).
22.Nishikawa, K. C. et al. Is titin a ‘winding filament’? A new twist on muscle contraction. Proceedings of the Royal Society B: Biological Sciences 279, 981–990 (2011).
23.Hessel, A. L., Lindstedt, S. L. & Nishikawa, K. C. Physiological Mechanisms of Eccentric Contraction and Its Applications: A Role for the Giant Titin Protein. Frontiers in Physiology 8, (2017).
24.Hyldahl, R. D. & Hubal, M. J. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle & Nerve 49, 155–170 (2013).
25.Roig, M. et al. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. British Journal of Sports Medicine 43, 556–568 (2008).
26.Isner-Horobeti, M.-E. et al. Eccentric Exercise Training: Modalities, Applications and Perspectives. Sports Medicine 43, 483–512 (2013).
27.Friedmann-Bette, B. et al. Effects of strength training with eccentric overload on muscle adaptation in male athletes. European Journal of Applied Physiology 108, 821–836 (2009).
28.Coffey, V. G. & Hawley, J. A. The Molecular Bases of Training Adaptation. Sports Medicine 37, 737–763 (2007).
29.Schoenfeld, B. J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. Journal of Strength and Conditioning Research 24, 2857–2872 (2010).
30.Harriman, D. G. F. The histochemistry of reactive masticatory muscle hypertrophy. Muscle & Nerve 19, 1447–1456 (1996).
31.Mackey, A. L. & Kjaer, M. The breaking and making of healthy adult human skeletal muscle in vivo. Skeletal Muscle 7, (2017).
32.Eliasson, J. et al. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. American Journal of Physiology-Endocrinology and Metabolism 291, E1197–E1205 (2006).
33.Toigo, M. & Boutellier, U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. European Journal of Applied Physiology 97, 643–663 (2006).
34.Godard, M. P., Wygand, J. W., Carpinelli, R. N., Catalano, S. & Otto, R. M. Effects of Accentuated Eccentric Resistance Training on Concentric Knee Extensor Strength. Journal of Strength and Conditioning Research 12, 26–29 (1998).
35.Seger, J. Y., Arvidsson, B., Thorstensson, A. & Seger, J. Y. Specific effects of eccentric and concentric training on muscle strength and morphology in humans. European Journal of Applied Physiology 79, 49–57 (1998).
36.Franchi, M. V. et al. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiologica 210, 642–654 (2014).
37.Hortobagyi, T. et al. Adaptive responses to muscle lengthening and shortening in humans. Journal of Applied Physiology 80, 765–772 (1996).

