26 de February de 2021
What’s fatigue?
Fatigue can be understood as a reversible and temporary loss of voluntary force production capacity (1). Depending on its duration we can understand it as chronic or acute fatigue. While acute fatigue can be improved with some changes in rest and lifestyle habits. The same is not true for chronic fatigue. Chronic fatigue can last for more than 1 month. In fact, residual systemic fatigue continues with overtraining, defined as a state of high stress, a failure to adapt to a training load or a decline in performance. Moreover, this overtraining may have a parasympathetic or sympathetic predominance depending on the training regime followed (2). Both forms of fatigue involve central and peripheral phenomena (2) although the mechanisms involved are not yet entirely clear.
Peripheral fatigue
Training with loads can lead to depletion of glycogen stores, CRP, ATP and an increase in inorganic phosphate and hydrogen levels. These changes are especially high when the intensity is close to 1RM (2). This would lead to increased fatigue and reduced muscle function.
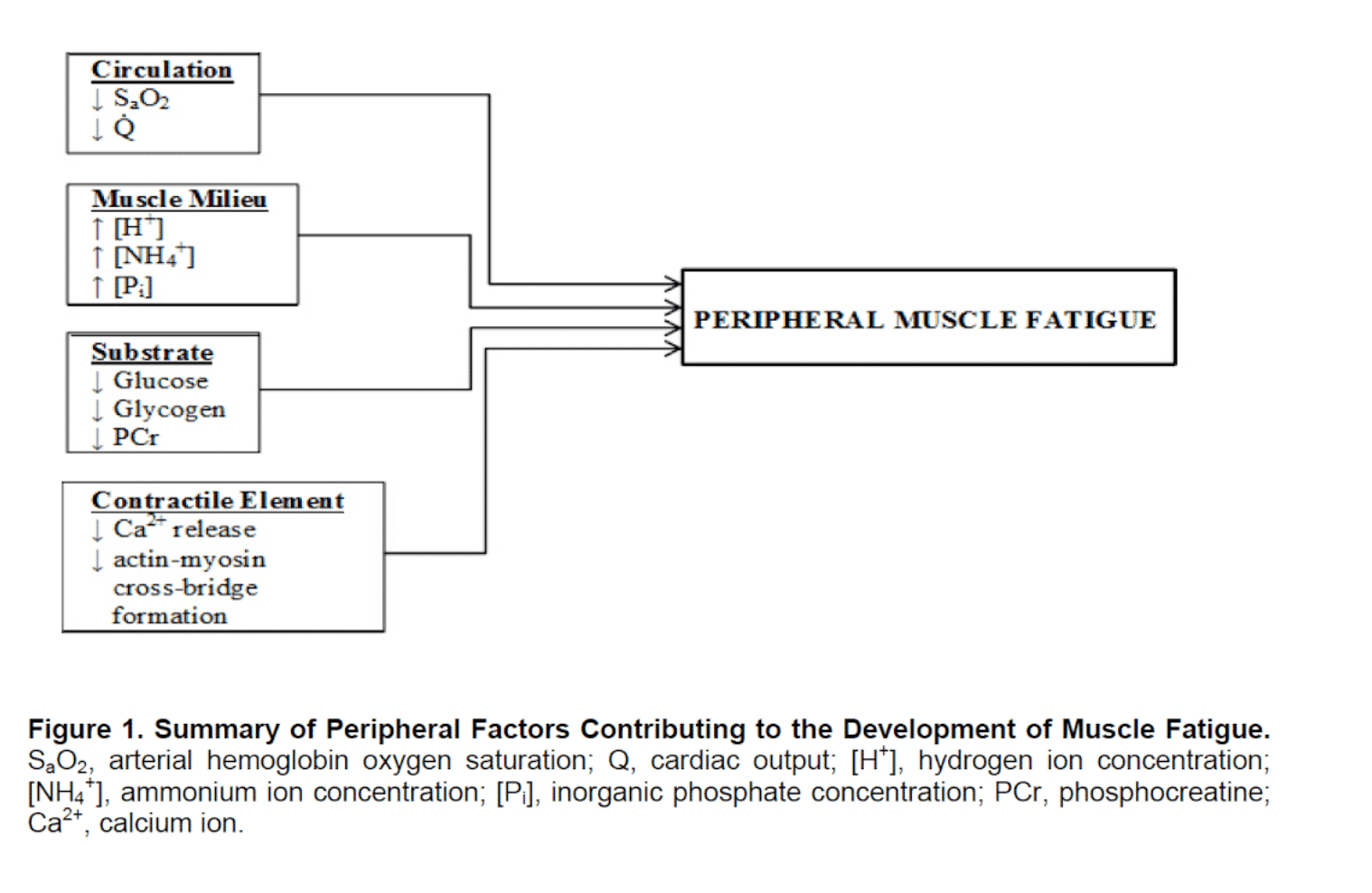
Muscle environment and energy substrates:
We found evidence that lowering Ph in muscle medium could delay the onset of fatigue as, elevated acidosis does not reduce glycogenolysis/glycolysis or inhibit energy metabolism in muscle fibres (4).
Instead, inorganic phosphate from CRP hydrolysis appears to be the primary cause of the onset of fatigue (5). In fact, in humans, the relationship between reduced Ph and the inability to maintain muscle function during the onset of fatigue does not always occur. Sometimes, strength recovers before Ph after fatiguing contractions (5).
Important evidence in favour of acidosis as a cause of reduced force production capacity was conducted on muscle fibres at a temperature of 15°C (6). But recent studies have focused on the temperature dependence of Ph effects on force.
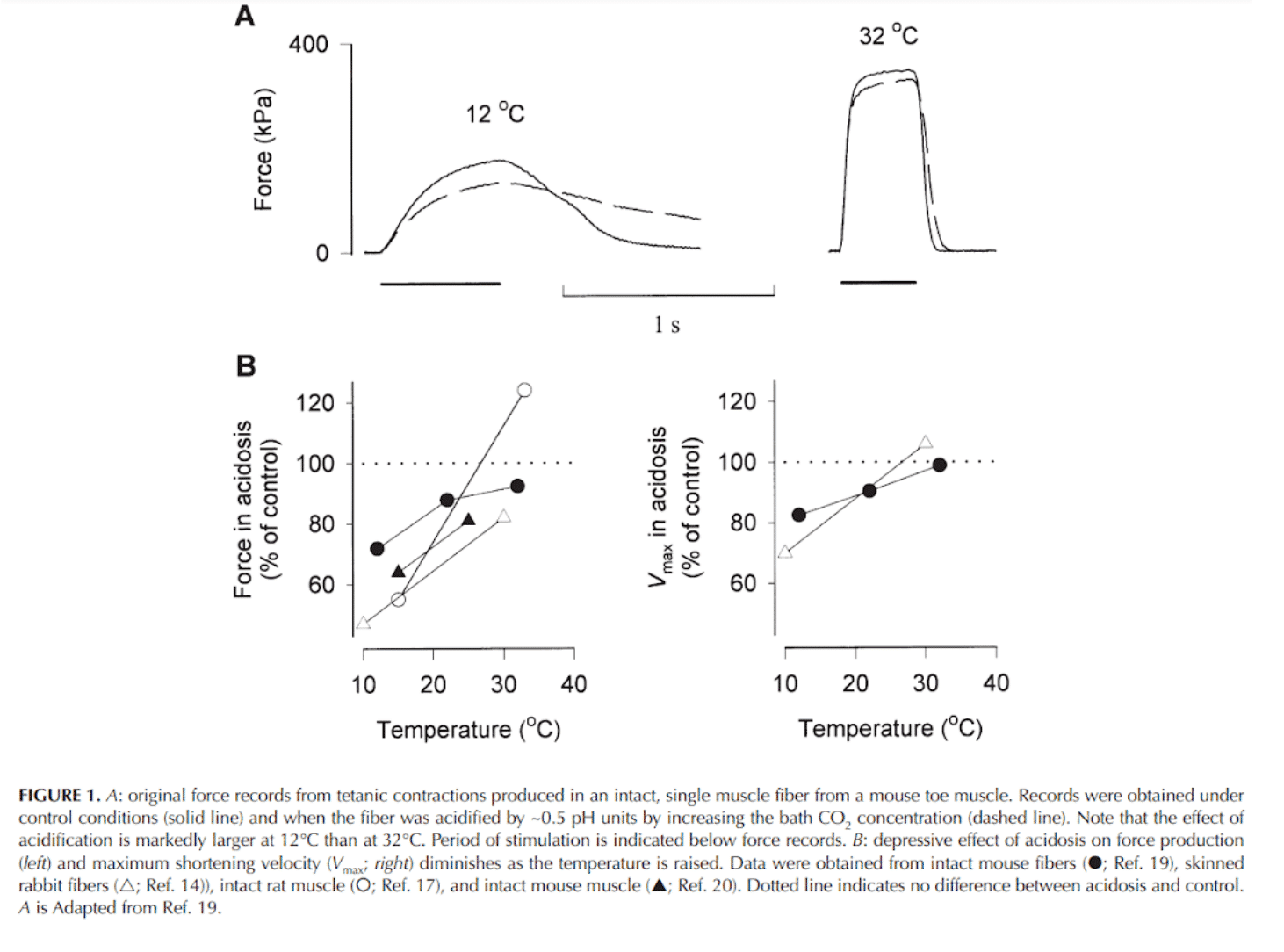
While at 10°C acidosis produced significant decreases in the ability to produce force, at 30°C this is not the case. In fact, at physiological temperature, acidosis may produce greater tetanic contractions (7).
In vitro acidosis can lead to inhibition of phosphorylase and phosphofructokinase (the latter catalyses the rate-limiting reaction of glycolysis, the switch from 6-P- to 1,6-diP-glucose) and thus to a reduction in ATP production capacity. This decrease in ATP production in vitro leads to a reduction in cross-bridge formation and Ca2+ pumping by the sarcoplasmic reticulum. However, at physiological temperature acidosis does not appear to accelerate the fatigue process (5).
Therefore, if acidosis is involved in the fatigue process, the effect could be indirect, and also via activation of type 3 and 4 nerve afferents (5).
Pi accumulation has been linked to a decrease in strength through direct action on cross-bridge action and reduced calcium sensitivity. In addition, high Pi concentrations would also affect the mechanism of calcium release from the sarcoplasmic reticulum (5).
During a first phase of fatigue, it increases Ca2+ release by increasing tetanic [Ca2+] force (5) Inhibits Ca2+ reabsorption leading to increases in tetanic strength [Ca2+] at first. But in the long term, Ca2+ would accumulate in other organelles or leave the cell. Therefore, the Ca2+ available for release decreases and force production capacity may decrease (5).
Contractile elements:
Calcium release is regionally disabled. However, the ability to produce force not only decreases due to the inability to release calcium, but also due to a decrease in calcium sensitivity (3).
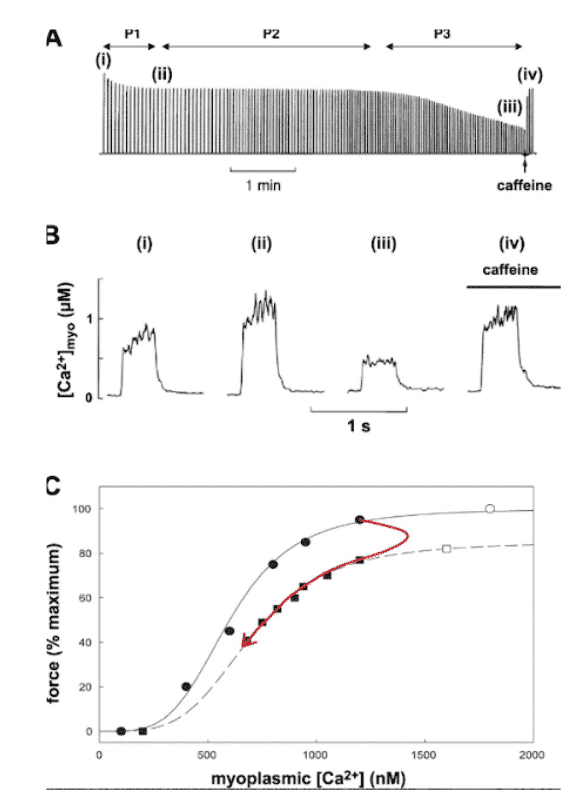
P1 indicates the first phase where there is a rapid and small decrease in force production. P2 indicates the second phase where there is little change in force production. P3 indicates the third phase where the decrease in force production is already pronounced. These phenomena are due to different physiological processes, i.e. P1 is due to a decrease in calcium ion concentration, P2 to a decrease in calcium ion sensitivity and P3 to Ca2+-activated peak force.
The levels of K+ ions and caffeine are able to facilitate this release. As we can see, 10 mg of caffeine is able to restore Ca2+ concentration to that of P2.
Muscle contraction depends on action potentials that lead to an adequate release of Ca2+ ions. Each action potential propagates rapidly across the muscle fibre membrane and into the tubular system where it is detected by voltage-sensitive molecules (dihydropyrin receptors) that open Ca2+ -releasing ryanodine receptors. The propagation of action potentials requires the activation of sodium-dependent channels (sodium potassium pump) (3). Each action potential requires a small influx of Na+ into the muscle fibre, and subsequent repolarisation requires K+ . Repeated contraction generates high concentrations of extracellular K+ and intracellular Na+, lowering their electrochemical gradient and decreasing or slowing the repolarisation capacity of the membrane (3).
There are many mechanisms responsible for regulating this process, but Cl- is the most important as the membrane is much more permeable to Cl- than to K+. As a consequence, the extracellular K+ concentration is not as high as previously thought and does not affect repolarisation as negatively (3). At rest, the inside of the cell is more negatively charged than the outside. As a consequence, if molecules with a positive electrochemical potential (Na+) are allowed to enter, we generate a more positive environment, leading to depolarisation (these channels are then inactivated). Repolarisation occurs when the K+ channels allow K+ to escape, generating a drop in the cell’s positive electrolyte potential, although it can also occur when Cl- enters. As Cl- is more permeable, the recovery of the negative electrolyte potential will be carried out mostly by Cl- entering the cell and excessive amounts of extracellular K+ will not accumulate (3).
Therefore, the decrease in pH related to muscle activity is not as decisive as previously thought. It may help maintain excitability by reducing Cl- conduction, allowing action potentials to propagate despite a high level of depolarisation and inactivation of Na+ channels. Moreover, low Ph levels inhibit Ca2+ retrieval by the sarcoplasmic reticulum allowing higher Ca2+ concentrations (more Ca2+ available for binding to troponin) (3).
The inability of the sarcoplasmic reticulum to release calcium is the cause of the final phase of fatigue (3).
Central fatigue
The motor cortex generates an electrical signal that is directed to the movement agonist muscles and the size of this signal is what determines motor recruitment when it reaches the muscle (8). Larger signals lead to greater recruitment. As motor recruitment is highly correlated with the number of active muscle fibres, it has a major impact on force production capacity (1).
Peripheral afferent feedback caused by both metabolite accumulation and inflammatory response may alter the size of the electrical signal (1).
In fact, during aerobic training it is greater when exercise duration is longer (greater inflammatory response), whereas central fatigue during strength training is greater when metabolite accumulation and fatigue sensation increase (accumulating volume near muscle failure and using light loads) (1).
Spinal cord:
After a repeated number of muscle contractions, the signal sent by the spinal cord starts to decrease relatively to the signal that is actually sent by the motor cortex (1). The causes of these phenomena have not yet been determined, but it is postulated that they could be mainly 3 (8)
- Response to afferent information from peripheral organs
- Inhibitory reflexes
- Signals from prefrontal cortex and cingulate cortex
Response to afferent information from peripheral organs:
Activation of nociceptive afferents type 3 and 4:
Peripheral fatigue could be a consequence of decreased ATP, glycogen and phosphocreatine levels. In addition to increased levels of inorganic phosphate and H-(8). These physiological phenomena occur mainly in sets carried to failure or 1RM lifts and lead to a reduction in muscle function through the activation of type 3 and 4 nociceptors, involved in the modulation of the central motor impulse.
Type 3 afferents are more mechanosensitive and are preferentially stimulated in response to force production. Type 4 afferents are more metabolo-sensitive than type 3. During muscle activity both types of afferents transmit information to the CNS and can inhibit muscle fibre contraction to avoid excessive fatigue or injury. Consequently, performance will be reduced.
The recruitment pattern at efforts close to 1RM follows the law of size. First low threshold fibres are recruited (type 1) and then high threshold fibres (type 2). But at intensities close to 1RM (muscle failure), hypoxia is generated, which means that these low-threshold O2-dependent fibres do not obtain the oxygen necessary to carry out the oxidative reactions that provide them with energy. This suggests that the activation of type 3 and 4 afferents occurs due to the activity of type 1 fibres.
Inhibitory reflexes:
Activation of 1 afferents.
I afferents are located in the Golgi tendon organ and report the tension produced by the contraction of motor units after high intensity contractions. The sensitivity of the organ to stretch has been shown to decrease by 15-30 seconds and the rate of unloading decreases by up to 50% for the same force production. In other words, for the same maximal voluntary contraction, while the force decreases, the unloading speed will be lower than for a non-fatigued muscle (9).
It seems to be that this first response in which the unloading frequency is reduced. But this decrease is followed by a subsequent increase with the development of fatigue. This also occurs in the H-reflex. (10) In fact, with the onset of central fatigue, the input of IⲀ-receptors to the motor-Ⲁ-neuron increases. That is, there is an increase in the excitability of the muscle spindles, and, this gain occurs in the presence of central fatigue. This stimulation occurs at the spinal level and not in higher centres of the brain (10).
Signals from the prefrontal cortex and cingulate:
During prolonged maximal contractions there is a decrease in force production and electromyographic (EMG) activation. This could be due to a reduced CNS impulse to the muscle, a decrease in discharge frequency, a reduction in active motor neurons and a decrease in discharge velocity, or a combination of both (12). However, the evidence so far on motor de-recruitment is still inconclusive.
Decreased dopamine levels and increased serotonin levels:
Dopamine levels increase with prolonged exercise, but return to their resting values with fatigue. In turn, serotonin (5-HT) levels increase with prolonged exercise (8). In addition, administration of a 5-HT receptor agonist reduces time to exhaustion. Conversely, administration of a 5-HT receptor antagonist increases the time to exhaustion. And in this case, variables such as body temperature, blood glucose, muscle and liver glycogen and stress hormone levels did not appear to change the development of fatigue following administration of the receptor agonists and antagonists, leading the researchers to believe that the development of fatigue resulted from changes in brain activity (8).
Both the mechanisms leading to central fatigue and peripheral fatigue do not affect the reduction in the ability to exert force in the same way.
The greatest reduction in power capacity is caused by the accumulation of metabolites and the inability to generate cross-bridges. These mechanisms last only a few hours and occur in training with sets taken to muscle failure or very close to it (11).
The reduction in the ability to exert force caused by muscle damage generated by both mechanical stress and repair mechanisms (calpain activity, defective calcium release mechanism due to sarcoplasmic reticulum damage and leukocyte accumulation). Muscle damage can occur in excess in eccentric training, especially where eccentric contraction occurs at high velocity. Greater muscle damage also occurs in full range of motion contractions, which is one of the reasons why full range of motion exercises produce greater fatigue than partial range of motion exercises. This phenomenon is capable of extending up to 5 days post-training (11).
The reduction in the ability to exert force due to inhibitions in the excitation-contraction process. This is closely related to the above as it is highly dependent on the release of calcium ions which will be defective if the sarcoplasmic reticulum is damaged. It has a shorter duration than muscle damage (3-4 days) (11).
The reduction in force-generating capacity due to reduced voluntary activation by central mechanisms lasts around 2-3 days and may be due to the inflammatory response following training (11).
Reduced sarcolemmal excitability also leads to reductions in force-generating capacity, but only lasts for a few hours after training (11).
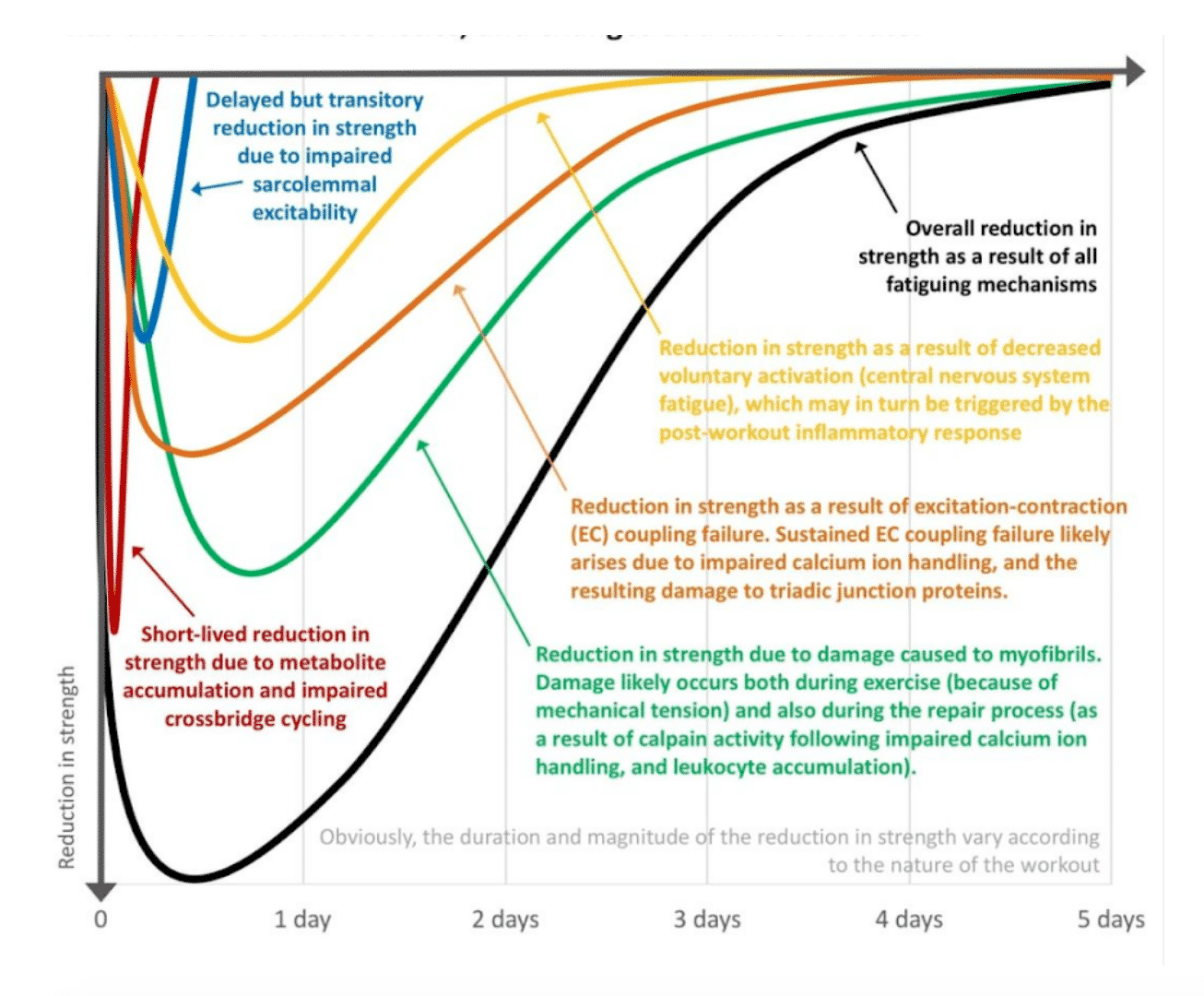
Chris Beardsley. Mechanisms involved in reduced force production
After analysing this we know that central fatigue is quite transient except when it occurs due to excessive muscle damage. Thus, large volumes can generate greater increases in peripheral fatigue than smaller volumes at higher intensity. In fact, if the muscle damage produced by a workout is low, central fatigue will hardly be observed (11).
Some types of strength training can cause excessive muscle damage, especially when exercises other than those previously performed are included. It will be the amount of muscle damage that will primarily determine how long it will take for strength levels to return (11).
How can we observe thin in the encoder?
Despite all this, it is very difficult to detect where the fatigue comes from, and we will probably always find both types occurring at the same time, albeit to different extents. The cause of the fatigue may be an excessively high training volume or an excessive amount of conditioning.
It is true, however, that although we cannot know exactly where this fatigue comes from, it will affect the ability to produce power and maximal strength in different ways. Thus, the onset of fatigue may be associated with a decrease in force production capacity at high speeds, while force production at low speeds is not so affected (13). In fact, high training volumes correlated with improvements in 1RM that could be explained by an increase in muscle mass and consequently cross-sectional area, while lower volumes caused greater gains in explosive movements such as vertical jumping. These improvements could be explained by a smaller shift from 2X to 2A fibres (14).
For all these reasons, it could be interesting to perform a multilevel monitoring of the same exercise. That is to say, to track the velocities of different loads belonging to different spectra of the force-velocity curve. For example, the velocity of a load belonging to the maximum force spectrum and another load belonging to the speed-force spectrum. In addition, you can combine this with other measurements of sport-specific explosive actions and observe whether there is actually a decrease in performance.
This can help you determine whether the lack of performance improvement is due to a period of overtraining or a period of detraining, as sometimes programmes lack the intensity to produce improvements.
How should you do multilevel monitoring?
First, choose the most relevant loads for that sport discipline and that really provide the data that will allow you to draw the right conclusions. Once you have chosen these loads, you should track the speed at which they are lifted throughout the different training blocks. This is a monitoring that you can do practically every day and that can not only give you an insight into the fatigue you are generating, but you can also observe the adaptations that are occurring in your athlete, but this is a completely different topic. First of all, you should avoid certain situations:
- Warm up properly, a poor warm up can show slower speeds. Make sure to perform explosive actions with maximum intentionality before starting the test.
- Avoid fatigue situations before the start of the test and during the warm-up. Give your athletes the necessary time to recover.
- Do not vary the load you track during the different blocks. We are interested in how the speed at which the load is lifted changes.
Bibliography:
- Chris Beardsley. What are the different types of fatigue?. Chris Beardsley. Apr, 19. 2020. Disponible en https://medium.com/@SandCResearch/what-are-the-different-types-of-fatigue-a631442c973d
- Zajac A, Chalimoniuk M, Maszczyk A, Golás A, Lngfort J. Central and Peripheral Fatigue During Resistance Exercise – A Critical Review. J Hum Kinet. 2015 Dec 22; 49: 159–169. DOI: 10.1515/hukin-2015-0118
- Allen DG, Lamb DG, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol (1985). 2008 Jan;104(1):296-305. Epub 2007 Oct 25.
- Graham D. Lamb, D. George Stephenson. Point:Counterpoint: Lactic acid accumulation is an advantage/disadvantage during muscle activity. J Appl Physiol 100: 1410 –1414, 2006; doi:10.1152/japplphysiol.00023.2006.
- Westerblad H, G. Allen D,2 and Lännergren J. Muscle Fatigue: Lactic Acid or Inorganic Phosphate the Major Cause?. News Physiol. Sci. Volume 17. February 2002
- Pate E, Bhimani M, Franks-Skiba K, and Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol (Lond) 486: 689694, 1995.
- Ranatunga KW. Effects of acidosis on tension development in mammalian skeletal muscle. Muscle Nerve 10: 439445, 1987.
- Shei RJ, Mickleborough TD. Relative Contributions of Central and Peripheral Factors in Human Muscle Fatigue during Exercise: A Brief Review. December 2013. Journal of Exercise Physiology Online 16(6):1-17
- Taylor JL, Et Al. Changes in muscle afferents, motoneurons and motor drive during muscle fatigue. November 2000. European Journal of Applied Physiology 83(2-3):106-15
- A. Biro, L. GriYn, E. Cafarelli. Reflex gain of muscle spindle pathways during fatigue. Exp Brain Res (2007) 177:157–166. DOI 10.1007/s00221-006-0656-7
- Chris Beardsley. What is recovery?. Chris Beardsley. 5 March. 2018. Disponible en https://medium.com/@SandCResearch/what-is-recovery-e76fb61bb5de
- Nonlinear cortical modulation of muscle fatigue: A functional MRI study. May 2003. Brain Research 957(2):320-9. DOI: 10.1016/S0006-8993(02)03665-X
- Vikmoen, O., Raastad, T., Ellefsen, S. et al. Adaptations to strength training differ between endurance-trained and untrained women. Eur J Appl Physiol 120, 1541–1549 (2020). https://doi.org/10.1007/s00421-020-04381-x
- Methenitis S, Mpampoulis T, Spiliopoulou P, Papadimas G, Papadopoulos C, Chalari E, Evangelidou E, Stasinaki AN, Nomikos T, Terzis G. Muscle fiber composition, jumping performance, and rate of force development adaptations induced by different power training volumes in females. Appl Physiol Nutr Metab. 2020 Sep;45(9):996-1006. doi: 10.1139/apnm-2019-0786. Epub 2020 Mar 23. PMID: 32203677.

