1 de June de 2020
Physical Inactivity and Covid-19: The Ignored Risks – Part II
Now that you’ve had time to assimilate the introduction about The Ignored Risks of Physical Inactivity and Covid-19 (you thought that was it?), let’s get deeper into what brought you here in the first place.
Physical inactivity and muscle mass
The preservation of muscle mass requires a constant supply of mechanical stimuli that directly or indirectly stimulate protein synthesis80. When we stop training, these essential stimuli required for muscle anabolism are removed and the balance between protein synthesis and protein degradation is broken down to degradation. Within a few days, objective signs of muscle atrophy can be found. In fact, significant losses of the quadriceps have been reported after only 2 days of leg immobilization (1.7%) 81 or 5 days of bed rest (2%) 82, which is associated with an even greater loss of muscle strength (8-9%) 82-84. During the following days and weeks, muscle atrophy is able to progress at an inexorable rate, 6% after 10 days 78, 10% after 29 days 85, 13% after 60 days 82, reaching 18% after 90 days 86. This rate of muscle atrophy grows exponentially, predicting a loss of muscle mass of approx. 8% in 30 days and ∼15% in 60 days.
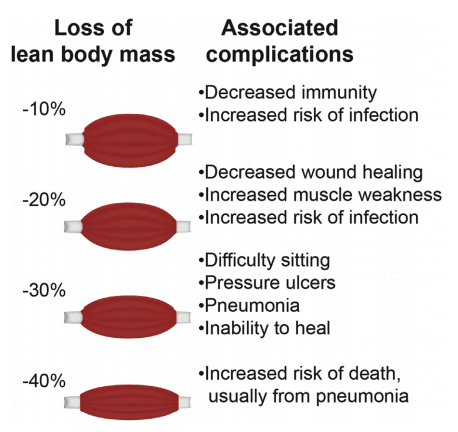 Figure 8: Complications of loss of lean body mass (muscle)87
Figure 8: Complications of loss of lean body mass (muscle)87
A recent survey conducted on the impact of sedentarism in 6733 people aged 18 to 98 years showed a clear association between physical activity, lean mass (muscle) and body fat 88. In addition, when comparing the muscle mass and function of sedentary people aged 20-80 with their counterparts in an athletic population, it is clear that maintaining a high level of physical activity preserves muscle mass and function throughout life.89
This benefit translates into a gain of approximately 20 to 25 years in terms of biological age when comparing the muscle mass and performance of 3-age vs. sedentary athletes 89.90. Similarly, people trained over a lifetime show 30% more muscle strength compared to sedentary people of the same age 91. Surprisingly, the benefits of leading an active lifestyle protect not only against loss of muscle mass and strength, but also appear to protect against the progressive muscle denervation that accompanies the aging process and is exacerbated by inactivity 92
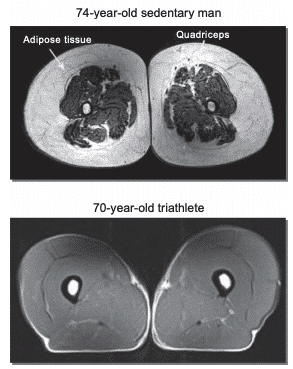 Figure 9: Nuclear magnetic resonance showing differences in subcutaneous fat tissue between a 74-year-old sedentary man versus a 70-year-old triathlete93
Figure 9: Nuclear magnetic resonance showing differences in subcutaneous fat tissue between a 74-year-old sedentary man versus a 70-year-old triathlete93
Muscle loss associated with aging, disuse and certain pathological conditions can lead to adverse health and quality of life deterioration. The loss of muscle mass, strength and function has not only local but also systemic adverse consequences that lead to physical, functional and congenital disability.87
Physical Inactivity and Metabolic Control
The current situation will reduce activity to levels well below the daily recommendation of 7500-10,000 steps per day, exacerbating the health problems resulting from physical inactivity 55.94. It is important to note that the negative health effects can be seen relatively quickly (3-14 days) when the decline in activity is accentuated, as will be the case worldwide in the current pandemic.95
Additional evidence reports that 2 weeks of reduced physical activity (from > 3500 to <1500 steps/day) in healthy older people (> 65 years and typically the most inactive proportion of the population) induced a small increase but measurable in insulin resistance and a reduction in the postprandial rate of muscle protein synthesis.96
Sedentary activities such as desk work, watching television, sitting are associated with increased mortality from all causes and increased morbidity (metabolic syndrome, cardiovascular disease) 97-99. The association is summarised in a recent review which concluded: “Higher levels of total physical activity, at any intensity, and shorter time spent sedentary are associated with a substantially reduced risk of premature mortality, with evidence of a non-linear dose-response pattern in middle-aged adults and older 100″.
The mechanisms through which inactivity results in decreased systemic insulin sensitivity and glucose intolerance are closely related to modifications within skeletal muscle 101-104.
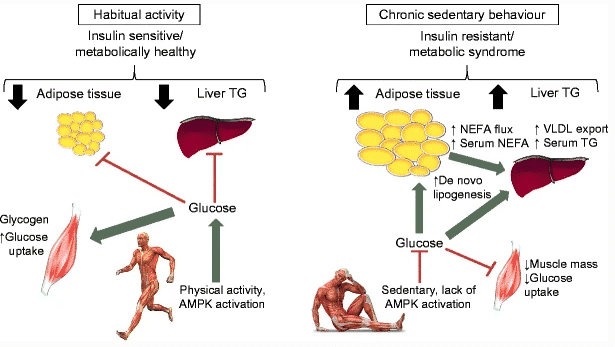 Figure 10: Being habitually active promotes the activation of metabolic pathways related to glucose uptake in skeletal muscle; thus, insulin sensitivity is preserved and less glucose is diverted to metabolic unfavorable stores 105.
Figure 10: Being habitually active promotes the activation of metabolic pathways related to glucose uptake in skeletal muscle; thus, insulin sensitivity is preserved and less glucose is diverted to metabolic unfavorable stores 105.
Evidence suggests that 7-10 days of bed rest in healthy individuals leads to a 10-34% decrease in insulin sensitivity throughout the body 102,106. However, decreased insulin sensitivity measured by the arteriovenous balance in the forearm 102 or leg 107 shows much greater results (47. -75%). This difference is due to the fact that the forearm and leg consist mainly of skeletal muscle, emphasizing the fundamental role of muscle in inactivity-induced insulin resistance, which appears to be attributable to reduced muscle contraction per se108.
Decreased insulin sensitivity with physical inactivity is not directly related to changes in body composition (loss of muscle mass, increased percentage of body fat) as this develops rapidly (within a few days) and well before muscle atrophy and/or increased body fat (or ectopic fat deposition) is established 109.
In times of restrictions due to the COVID19 pandemic, it is important to realize that a moderate amount of daily exercise of sufficient intensity is necessary 110.
Physical Inactivity and Cardiorespiratory Impact
VO2max is the maximum capacity to take oxygen from the environment in the lungs, transport it in the blood and use it by the body, mainly in the muscles, and therefore it is considered a variable that evaluates the maximum performance of the cardiorespiratory system and the skeletal muscles in the transport and use of O2111. Besides being one of the main determinants of exercise tolerance, VO2max is considered an index of “cardiorespiratory fitness”. In both healthy subjects and patients with cardiovascular disease, “exercise capacity is one of the strongest predictors of mortality, even more so than other established risk factors for cardiovascular disease “112. According to the same authors, for every 1 MET, the decrease in mortality due to cardiorespiratory fitness increases by 12% 112.
Saltin et al, one of the fathers of exercise physiology, in 1968, showed that individuals lose an average of 28% of maximum oxygen consumption (VO2max) and 11% of cardiac volume after a period of 20 days in bed113 .
Saltin et al, one of the fathers of exercise physiology, in 1968, showed that individuals lose an average of 28% of maximum oxygen consumption (VO2max) and 11% of heart volume after a period of 20 days in bed113 .
In line with these data, a recent study “anticipated” in much the same way the context to which hundreds of millions of people worldwide are now exposed as a result of confinement to the home. In that study, a group of young, healthy men sharply reduced the number of steps per day, from a baseline of ∼10,000 to ∼1350, and maintained this level of activity for 2 weeks.114 The effect, devastating, after 2 weeks, subjects had a ~ 7% decrease in VO2max114. Interestingly, the rate of decrease in VO2max reported was remarkably similar to the average rate of decrease in VO2max observed in the bed rest studies115 . If we assume that the rate of decrease in VO2max is also linear after forced inactivity not associated with bed rest (like the confinement by COVID-19), over a period of 2 months, the VO2max would decrease by approximately 30%, a not very real and speculative number but enough to try to prevent the worst possible scenario from becoming a reality.
As for older subjects, the percentage decreased of VO2max during 2 weeks of bed rest was twice as high in subjects aged 60 (-15%) compared to that observed in younger controls116 . Furthermore, during a 2-week post-bed rest period, young subjects would appear to recover the baseline VO2max, while the elderly recovery is less or even incomplete 116 complicating the picture for this population.
Therefore, in a hypothetical 70-year-old sedentary subject with a VO2max of ∼25 ml/kg/min-1, 4 weeks of forced inactivity would probably translate into a decrease of ∼15% in VO2max, corresponding to a decrease of ∼3.75 ml/kg/min-1, which is equivalent to ∼1 METs: Which, in turn, would translate into an increase of ∼12% in mortality.
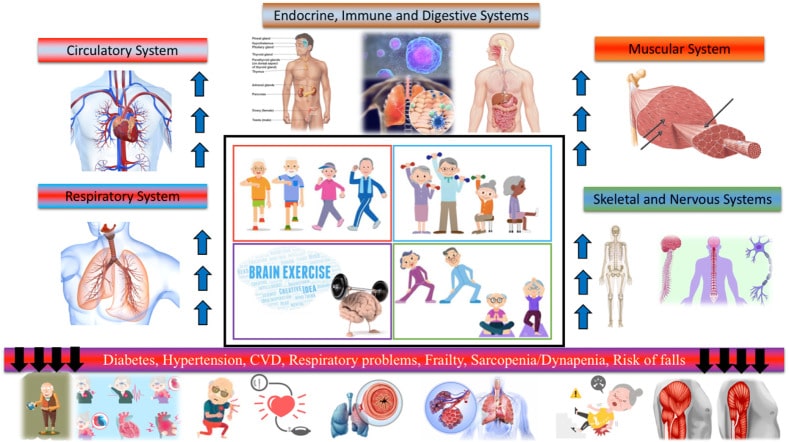 Figure 11: Physical exercise improves the health of older people by acting on different systems and organs117.
Figure 11: Physical exercise improves the health of older people by acting on different systems and organs117.
Finally, there would appear to be a direct dose-response relationship between the “volume” of exercise (duration x intensity) and cardiorespiratory fitness. Approximately 50% of the protective effects of physical activity are explained by a reduction in traditional cardiovascular risk factors, such as high blood pressure and blood lipids55. Other protective effects are presumably related to a decrease in low-grade inflammation of visceral fat tissue and a decrease in insulin resistance55.
Conclusions
The evidence that exercise is critical to preserve health and quality of life is indisputable as is the damaging impact of the COVID-19 pandemic on sedentary and inactive people 117,118. It is important to note that we may also be at risk of a vicious cycle where current and potentially accelerated patterns of inactivity may magnify the impact of current and future pandemics. Not surprisingly, people infected with COVID-19 are much more likely to be hospitalized and have a worse prognosis if they have underlying medical conditions, such as chronic diseases, that affect the immune response. In addition, the evidence associating with a significantly increased risk of chronic disease in physically inactive people is strongly evidenced6,8,117,118. Ergo, the relationship between physical inactivity and risks of health complications and mortality rates associated with 19-COVID can no longer be ignored. If the prevalence of chronic conditions caused by unhealthy lifestyles were lower, would the catastrophic effects of the COVID-2019 pandemic be reduced?
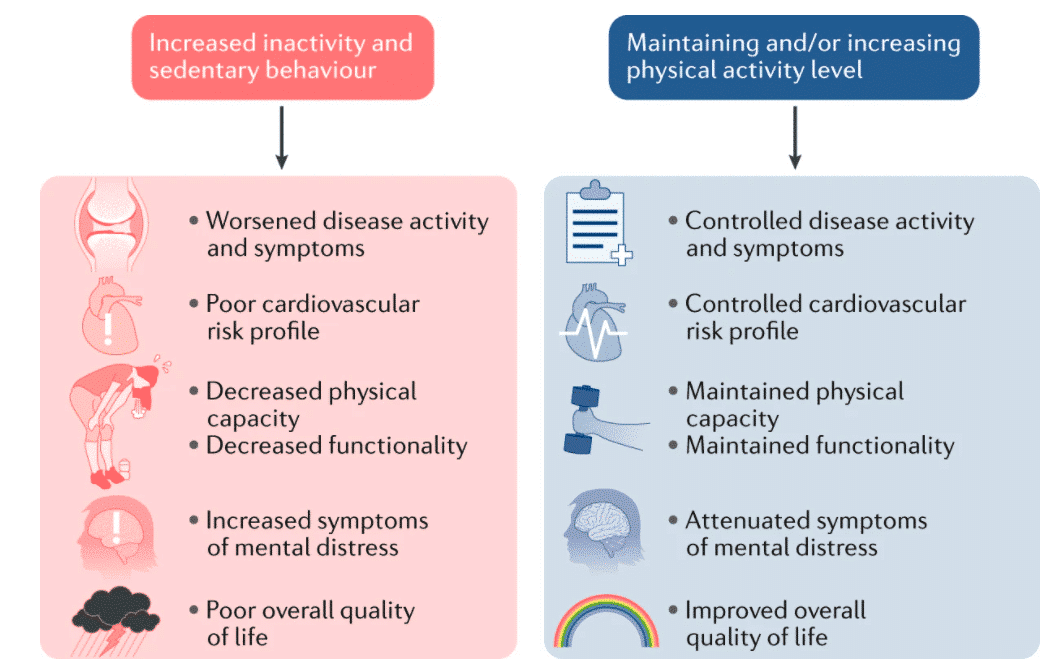 Figure 12: Physical inactivity and sedentary behavior can be detrimental to health, the cardiovascular risk profile, physical capacity and function, and mental health, resulting in a poor quality of life, while maintaining more optimal levels of physical activity can help improve these detrimental effects.119
Figure 12: Physical inactivity and sedentary behavior can be detrimental to health, the cardiovascular risk profile, physical capacity and function, and mental health, resulting in a poor quality of life, while maintaining more optimal levels of physical activity can help improve these detrimental effects.119
Therefore, during quarantine, being physically active is essential for mental and physical health. Fortunately, a wide range of exercises, such as aerobic exercise or strength training with or without equipment, taking advantage of new technologies that allow for guidance by appropriate professionals through video or application, can be performed in the home and should be encouraged. Along these lines, we encourage national, federal and regional governments around the world to include exceptions for physical activity in activity closures at the national level. These should also allow for outdoor physical activities (e.g., walking, running, or other individual sports), and thus prevent the COVID-19 pandemic from generating unfavorable consequences beyond the virus120.
References
1.Zhu, N. et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. New England Journal of Medicine 382, 727–733 (2020).
2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature microbiology 5, 536–544 (2020).
3.Parnell, D., Widdop, P., Bond, A. & Wilson, R. COVID-19, networks and sport. Managing Sport and Leisure 1–7 (2020). doi:10.1080/23750472.2020.1750100
4.Hammami, A., Harrabi, B., Mohr, M. & Krustrup, P. Physical activity and coronavirus disease 2019 (COVID-19): specific recommendations for home-based physical training. Managing Sport and Leisure 1–6 (2020). doi:10.1080/23750472.2020.1757494
5.Chen, P. et al. Coronavirus disease (COVID-19): The need to maintain regular physical activity while taking precautions. Journal of Sport and Health Science 9, 103–104 (2020).
6.Stevens, A. M. H. Lessons from covid-19: visiting patients at home and assessing comorbidities. BMJ m1385 (2020). doi:10.1136/bmj.m1385
7.Dayal, D. We urgently need guidelines for managing COVID‐19 in children with comorbidities. Acta Paediatrica (2020). doi:10.1111/apa.15304
8.Ebrahimi, M., Saki Malehi, A. & Rahim, F. COVID-19 Infection in Medical Staffs versus Patients: A Systematic Review and Meta-analysis of Laboratory Findings, Comorbidities, and Clinical Outcome. SSRN Electronic Journal (2020). doi:10.2139/ssrn.3580517
9.Hall, G., Laddu, D. R., Phillips, S. A., Lavie, C. J. & Arena, R. A tale of two pandemics: How will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Progress in Cardiovascular Diseases (2020). doi:10.1016/j.pcad.2020.04.005
10.Knipe, D. M. et al. Fundamental Virology, 4th Edition; and Fields Virology, 4th Edition, Volumes I and II:Fundamental Virology, 4th Edition;Fields Virology, 4th Edition, Volumes I and II. Clinical Infectious Diseases 34, 1029–1030 (2002).
11.Falsey, A. R. & Walsh, E. E. Novel coronavirus and severe acute respiratory syndrome. The Lancet 361, 1312–1313 (2003).
12.Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
13.Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 395, 507–513 (2020).
14.Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395, 497–506 (2020).
15.Yang, J. et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. International Journal of Infectious Diseases 94, 91–95 (2020).
16.Wu, Z. & McGoogan, J. M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA 323, 1239 (2020).
17.De Groot, R. J. et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. Journal of virology 87, 7790–2 (2013).
18.Zaki, A. M., van Boheemen, S., Bestebroer, T. M., Osterhaus, A. D. M. E. & Fouchier, R. A. M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. New England Journal of Medicine 367, 1814–1820 (2012).
19.An, P., Song, P., Wang, Y. & Liu, B. Asymptomatic Patients with Novel Coronavirus Disease (COVID-19). Balkan Medical Journal (2020). doi:10.4274/balkanmedj.galenos.2020.2020.4.20
20.Dahl, E. Coronavirus (Covid-19) outbreak on the cruise ship Diamond Princess. International Maritime Health 71, 5–8 (2020).
21.Zhang, S. et al. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: A data-driven analysis. International Journal of Infectious Diseases 93, 201–204 (2020).
22.Russell, T. W. et al. Estimating the infection and case fatality ratio for coronavirus disease (COVID-19) using age-adjusted data from the outbreak on the Diamond Princess cruise ship, February 2020. Eurosurveillance 25, (2020).
23.Salje, H. et al. Estimating the burden of SARS-CoV-2 in France. Science (New York, N.Y.) eabc3517 (2020). doi:10.1126/science.abc3517
24.Onder, G., Rezza, G. & Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA (2020). doi:10.1001/jama.2020.4683
25.Rezende, L. F. M. de, Rey-López, J. P., Matsudo, V. K. R. & Luiz, O. do C. Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health 14, (2014).
26.Banerjee, A. et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. The Lancet (2020). doi:10.1016/s0140-6736(20)30854-0
27.Fehr, A. R. & Perlman, S. Coronaviruses: an overview of their replication and pathogenesis. Methods in molecular biology (Clifton, N.J.) 1282, 1–23 (2015).
28.Weiss, S. R. & Navas-Martin, S. Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus. Microbiology and Molecular Biology Reviews 69, 635–664 (2005).
29.Perlman, S. Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses. PLOS Pathogens 11, e1005023 (2015).
30.Song, Z. et al. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses 11, 59 (2019).
31.Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C. & Garry, R. F. The proximal origin of SARS-CoV-2. Nature medicine 26, 450–452 (2020).
32.Cui, J., Li, F. & Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology 17, 181–192 (2018).
33.Decaro, N. & Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Veterinary Microbiology 244, 108693 (2020).
34.Majumder, M. & Mandl, K. D. Early Transmissibility Assessment of a Novel Coronavirus in Wuhan, China. SSRN Electronic Journal (2020). doi:10.2139/ssrn.3524675
35.Lauer, S. A. et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Annals of Internal Medicine 172, 577–582 (2020).
36.Wang, D. et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 323, 1061 (2020).
37.Zhang, H. et al. Clinical characteristics of 194 cases of COVID-19 in Huanggang and Taian, China. Infection (2020). doi:10.1007/s15010-020-01440-5
38.Wang, W., Tang, J. & Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019‐nCoV) in Wuhan, China. Journal of Medical Virology 92, 441–447 (2020).
39.Li, W. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 (2003).
40.Jia, H. P. et al. ACE2 Receptor Expression and Severe Acute Respiratory Syndrome Coronavirus Infection Depend on Differentiation of Human Airway Epithelia. Journal of Virology 79, 14614–14621 (2005).
41.Walls, A. C. et al. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181, 281–292.e6 (2020).
42.Letko, M., Marzi, A. & Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature Microbiology 5, 562–569 (2020).
43.Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology (2020). doi:10.1038/s41577-020-0311-8
44.Qin, C. et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clinical Infectious Diseases (2020). doi:10.1093/cid/ciaa248
45.Zhou, Y. et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. National Science Review (2020). doi:10.1093/nsr/nwaa041
46.Tian, S. et al. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. Journal of Thoracic Oncology 15, 700–704 (2020).
47.Small, B. A. et al. CD8+ T Cell–mediated Injury In Vivo Progresses in the Absence of Effector T Cells. Journal of Experimental Medicine 194, 1835–1846 (2001).
48.Liu, S. et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Scientific Reports 6, (2016).
49.Matthews, C. E. et al. Amount Of Time Spent In Sedentary Behaviors And Cause-specific Mortality In Us Adults. Medicine & Science in Sports & Exercise 43, 28 (2011).
50.Wilmot, E. G. et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 55, 2895–2905 (2012).
51.KATZMARZYK, P. T., CHURCH, T. S., CRAIG, C. L. & BOUCHARD, C. Sitting Time and Mortality from All Causes, Cardiovascular Disease, and Cancer. Medicine & Science in Sports & Exercise 41, 998–1005 (2009).
52.Stamatakis, E., Hamer, M. & Dunstan, D. W. Screen-Based Entertainment Time, All-Cause Mortality, and Cardiovascular Events. Journal of the American College of Cardiology 57, 292–299 (2011).
53.Hu, F. B. Television Watching and Other Sedentary Behaviors in Relation to Risk of Obesity and Type 2 Diabetes Mellitus in Women. JAMA 289, 1785 (2003).
54.FORD, E. S. et al. Television watching and incident diabetes: Findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. Journal of Diabetes 2, 23–27 (2010).
55.Booth, F. W., Roberts, C. K., Thyfault, J. P., Ruegsegger, G. N. & Toedebusch, R. G. Role of Inactivity in Chronic Diseases: Evolutionary Insight and Pathophysiological Mechanisms. Physiological Reviews 97, 1351–1402 (2017).
56.Lee, I.-M. et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet 380, 219–229 (2012).
57.Booth, F. W., Roberts, C. K. & Laye, M. J. Lack of Exercise Is a Major Cause of Chronic Diseases. Comprehensive Physiology (2012). doi:10.1002/cphy.c110025
58.Ruiz-Casado, A. et al. Exercise and the Hallmarks of Cancer. Trends in Cancer 3, 423–441 (2017).
59.LI, R. et al. Associations of Muscle Mass and Strength with All-Cause Mortality among US Older Adults. Medicine & Science in Sports & Exercise 50, 458–467 (2018).
60.Lang, P. O., Mitchell, W. A., Lapenna, A., Pitts, D. & Aspinall, R. Immunological pathogenesis of main age-related diseases and frailty: Role of immunosenescence. European Geriatric Medicine 1, 112–121 (2010).
61.Gleeson, M. et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature Reviews Immunology 11, 607–615 (2011).
62.AGRAWAL, A., AGRAWAL, S. & GUPTA, S. Dendritic cells in human aging. Experimental Gerontology 42, 421–426 (2007).
63.Montecino-Rodriguez, E., Berent-Maoz, B. & Dorshkind, K. Causes, consequences, and reversal of immune system aging. Journal of Clinical Investigation 123, 958–965 (2013).
64.Ikeno, Y., Orihuela, C. & Van Remmen, H. Inflammation in Aging and Age-related Disease. Pathobiology of Aging & Age-related Diseases 1, 14729 (2011).
65.Lee, W. et al. Long-term Moderate Exercise Improves T-cell Proliferation In Older Adults. Medicine & Science in Sports & Exercise 36, S228 (2004).
66.Lowder, T., Padgett, D. A. & Woods, J. A. Moderate exercise protects mice from death due to influenza virus. Brain, Behavior, and Immunity 19, 377–380 (2005).
67.Sun, Y. & Woods, J. A. Effects of Acute Eccentric Exercise on Immune Responses to Vaccination in Young and Aged Mice. Medicine & Science in Sports & Exercise 50, 394 (2018).
68.Shephard, R. J. Cardiovascular Exercise Training Extends Influenza Vaccine Seroprotection in Sedentary Older Adults: The Immune Function Intervention Trial. Yearbook of Sports Medicine 2010, 260–262 (2010).
69.Pascoe, A. R., Fiatarone Singh, M. A. & Edwards, K. M. The effects of exercise on vaccination responses: A review of chronic and acute exercise interventions in humans. Brain, Behavior, and Immunity 39, 33–41 (2014).
70.Kohut, M. L. et al. Corrigendum to ‘Moderate exercise improves antibody response to influenza immunization in older adults’ [Vaccine 22 (2004) 2298–2306]. Vaccine 23, 278 (2004).
71.Pedersen, B. K. & Febbraio, M. A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiological Reviews 88, 1379–1406 (2008).
72.Muñoz-Cánoves, P., Scheele, C., Pedersen, B. K. & Serrano, A. L. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS Journal 280, 4131–4148 (2013).
73.Welc, S. S. & Clanton, T. L. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Experimental Physiology 98, 359–371 (2012).
74.Wallace, D. L. et al. Prolonged exposure of naïve CD8+T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length. Immunology 119, 243–253 (2006).
75.Zbinden‐Foncea, H., Francaux, M., Deldicque, L. & Hawley, J. A. Does high cardiorespiratory fitness confer some protection against pro‐inflammatory responses after infection by SARS‐CoV‐2? Obesity (2020). doi:10.1002/oby.22849
76.Cuthbertson, D. P. The influence of prolonged muscular rest on metabolism. Biochemical Journal 23, 1328–1345 (1929).
77.Narici, M. et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. European Journal of Sport Science 1–22 (2020). doi:10.1080/17461391.2020.1761076
78.Narici, M. V. et al. Early Biomarkers of Muscle Atrophy and of Neuromuscular Alterations During 10‐Day Bed Rest. The FASEB Journal 34, 1–1 (2020).
79.Wilkinson, D. J., Piasecki, M. & Atherton, P. J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Research Reviews 47, 123–132 (2018).
80.Schoenfeld, B. J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. Journal of Strength and Conditioning Research 24, 2857–2872 (2010).
81.KILROE, S. P., FULFORD, J., JACKMAN, S. R., VAN LOON, L. J. C. & WALL, B. T. Temporal Muscle-specific Disuse Atrophy during One Week of Leg Immobilization. Medicine & Science in Sports & Exercise 52, 944–954 (2020).
82.Mulder, E. et al. Musculoskeletal effects of 5 days of bed rest with and without locomotion replacement training. European Journal of Applied Physiology 115, 727–738 (2014).
83.Demangel, R. et al. Early structural and functional signature of 3-day human skeletal muscle disuse using the dry immersion model. The Journal of Physiology 595, 4301–4315 (2017).
84.De Boer, M. D., Maganaris, C. N., Seynnes, O. R., Rennie, M. J. & Narici, M. V. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. The Journal of Physiology 583, 1079–1091 (2007).
85.Alkner, B. A. & Tesch, P. A. Efficacy of a gravity-independent resistance exercise device as a countermeasure to muscle atrophy during 29-day bed rest. Acta Physiologica Scandinavica 181, 345–357 (2004).
86.Alkner, B. A. & Tesch, P. A. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. European Journal of Applied Physiology 93, 294–305 (2004).
87.Argilés, J. M., Campos, N., Lopez-Pedrosa, J. M., Rueda, R. & Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. Journal of the American Medical Directors Association 17, 789–796 (2016).
88.Kyle, U. G., Morabia, A., Schutz, Y. & Pichard, C. Sedentarism affects body fat mass index and fat-free mass index in adults aged 18 to 98 years. Nutrition 20, 255–260 (2004).
89.Grassi, B., Cerretelli, P., Narici, M. V. & Marconi, C. Peak anaerobic power in master athletes. European Journal of Applied Physiology and Occupational Physiology 62, 394–399 (1991).
90.PEARSON, S. J. et al. Muscle function in elite master weightlifters. Medicine & Science in Sports & Exercise 34, 1199–1206 (2002).
91.AAGAARD, P., MAGNUSSON, P. S., LARSSON, B., KJÆR, M. & KRUSTRUP, P. Mechanical Muscle Function, Morphology, and Fiber Type in Lifelong Trained Elderly. Medicine & Science in Sports & Exercise 39, 1989–1996 (2007).
92.Mosole, S. et al. Long-Term High-Level Exercise Promotes Muscle Reinnervation With Age. Journal of Neuropathology & Experimental Neurology 73, 284–294 (2014).
93.Wroblewski, A. P., Amati, F., Smiley, M. A., Goodpaster, B. & Wright, V. Chronic Exercise Preserves Lean Muscle Mass in Masters Athletes. The Physician and Sportsmedicine 39, 172–178 (2011).
94.Trost, S. G., Blair, S. N. & Khan, K. M. Physical inactivity remains the greatest public health problem of the 21st century: evidence, improved methods and solutions using the ‘7 investments that work’ as a framework. British Journal of Sports Medicine 48, 169–170 (2014).
95.Tudor-Locke, C., Craig, C. L., Thyfault, J. P. & Spence, J. C. A step-defined sedentary lifestyle index: <5000 steps/day. Applied Physiology, Nutrition, and Metabolism 38, 100–114 (2013).
96.Breen, L. et al. Two Weeks of Reduced Activity Decreases Leg Lean Mass and Induces ‘Anabolic Resistance’ of Myofibrillar Protein Synthesis in Healthy Elderly. The Journal of Clinical Endocrinology & Metabolism 98, 2604–2612 (2013).
97.Shephard, R. J. Sitting Time and Mortality from All Causes, Cardiovascular Disease, and Cancer. Yearbook of Sports Medicine 2010, 124–125 (2010).
98.Van der Ploeg, H. P. Sitting Time and All-Cause Mortality Risk in 222 497 Australian Adults. Archives of Internal Medicine 172, 494 (2012).
99.Dunstan, D. W. et al. Associations of TV viewing and physical activity with the metabolic syndrome in Australian adults. Diabetologia 48, 2254–2261 (2005).
100.Ekelund, U. et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ l4570 (2019). doi:10.1136/bmj.l4570
101.Sonne, M. P. et al. Endothelial function after 10 days of bed rest in individuals at risk for type 2 diabetes and cardiovascular disease. Experimental Physiology 96, 1000–1009 (2011).
102.Sonne, M. P. et al. Effect of 10 days of bedrest on metabolic and vascular insulin action: a study in individuals at risk for type 2 diabetes. Journal of Applied Physiology 108, 830–837 (2010).
103.Arciero, P. J., Smith, D. L. & Calles-Escandon, J. Effects of short-term inactivity on glucose tolerance, energy expenditure, and blood flow in trained subjects. Journal of Applied Physiology 84, 1365–1373 (1998).
104.Alibegovic, A. C. et al. Impact of 9 Days of Bed Rest on Hepatic and Peripheral Insulin Action, Insulin Secretion, and Whole-Body Lipolysis in Healthy Young Male Offspring of Patients With Type 2 Diabetes. Diabetes 58, 2749–2756 (2009).
105.Bowden Davies, K. A. et al. Short-term decreased physical activity with increased sedentary behaviour causes metabolic derangements and altered body composition: effects in individuals with and without a first-degree relative with type 2 diabetes. Diabetologia 61, 1282–1294 (2018).
106.Stuart, C. A., Shangraw, R. E., Prince, M. J., Peters, E. J. & Wolfe, R. R. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism 37, 802–806 (1988).
107.Mikines, K. J., Richter, E. A., Dela, F. & Galbo, H. Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. Journal of Applied Physiology 70, 1245–1254 (1991).
108.Crossland, H., Skirrow, S., Puthucheary, Z. A., Constantin‐Teodosiu, D. & Greenhaff, P. L. The impact of immobilisation and inflammation on the regulation of muscle mass and insulin resistance: different routes to similar end‐points. The Journal of Physiology 597, 1259–1270 (2018).
109.Knudsen, S. H. et al. Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. Journal of Applied Physiology 113, 7–15 (2012).
110.Slentz, C. A., Houmard, J. A. & Kraus, W. E. Modest Exercise Prevents the Progressive Disease Associated with Physical Inactivity. Exercise and Sport Sciences Reviews 18–23 (2007). doi:10.1249/01.jes.0000240019.07502.01
111.BASSETT, D. R. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Medicine & Science in Sports & Exercise 70 (2000). doi:10.1097/00005768-200001000-00012
112.Myers, J. et al. Exercise capacity and mortality among men referred for exercise testing. ACC Current Journal Review 11, 33–34 (2002).
113.Response to exercise after bed rest and after training. American Heart Journal 78, 430 (1969).
114.Krogh-Madsen, R. et al. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. Journal of Applied Physiology 108, 1034–1040 (2010).
115.Ried-Larsen, M., Aarts, H. M. & Joyner, M. J. Effects of strict prolonged bed rest on cardiorespiratory fitness: systematic review and meta-analysis. Journal of Applied Physiology 123, 790–799 (2017).
116.Pišot, R. et al. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. Journal of Applied Physiology 120, 922–929 (2016).
117.Jiménez-Pavón, D., Carbonell-Baeza, A. & Lavie, C. J. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: Special focus in older people. Progress in Cardiovascular Diseases (2020). doi:10.1016/j.pcad.2020.03.009
- et al. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 — United States, February 12–March 28, 2020. MMWR. Morbidity and Mortality Weekly Report 69, 382–386 (2020).
119.Pinto, A. J., Dunstan, D. W., Owen, N., Bonfá, E. & Gualano, B. Combating physical inactivity during the COVID-19 pandemic. Nature Reviews Rheumatology (2020). doi:10.1038/s41584-020-0427-z
120.Lippi, G., Henry, B. M. & Sanchis-Gomar, F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19). European Journal of Preventive Cardiology 204748732091682 (2020). doi:10.1177/2047487320916823

