9 de January de 2023
RFD (Rate of Force Development) Curve and Velocity-Based Training
Neuromuscular strength is a physical capacity that has gained great relevance in the world of training in recent years. Where today strength training takes place 40 or 50 years ago, endurance training took place. Strength has gained so much weight in the training industry because it has been shown that other physical capabilities stem to a large extent from the ability we have to apply strength. In other words, the other capabilities depend on strength (1). Let’s take two totally different examples; on the one hand, we have a marathon runner whose main function is to run the 42 km in the shortest possible time. On the other hand, we have a 100-meter sprinter. The sprinter has the main characteristic of being very fast in a very short period of time and the faster the better. In the case of marathon runners, their main characteristic is endurance, since they must run for 2 hours without stopping at a high speed, which means having a brutal resistance. Both athletes must train different physical capacities for their sports modalities, however, a few years ago it was demonstrated that if both train in neuromuscular strength, both would improve their marks.
In other words, strength capacity and its training has taken the leading position in training because greater muscular strength means better overall performance. In the case of the sprinter, it will allow him to better support each of the steps he performs during the 100 meters because the greater the force, the greater the capacity to support the newtons generated at each moment of the race. And, on the other hand, gaining strength in the marathon runner will allow him to print a little more force in each step during the 42 km and be able to scratch a few seconds to the clock. Therefore, strength is a feature that is becoming more important every day and in today’s blog post we are going to make a special mention of it.
What is RFD and what is the force-time curve?
The force-time curve is the curve that defines the relationship between the force applied in an exercise and the time it takes to apply that force. When a person applies an overwhelming force, i.e., a force that cannot move, the force application goes from 0 to X Newtons in a very short time. Once the maximum force produced is reached, it should be maintained. However, if the test is done correctly the force we have produced is not maintained and gradually decreases. Below is an example of a force-time curve.
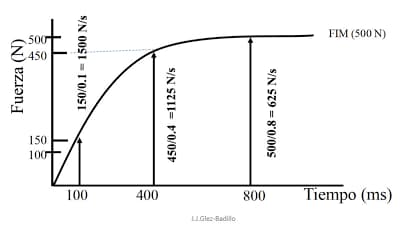
Source: https://www.ensasport.com/rfd-potencia-y-cde/
In the image we can see the time in milliseconds and the force application in newtons. The Rate of Force Development (RFD) refers to the relationship between the time and the force applied at the different points of this curve. The RFD can be given by the scale value at each point or by the slope formed by two points on the curve (2). Normally, it can be called RFD curve or simply RFD. The highest RFD occurs at the point at which the greatest force is applied per unit time. The RFD is usually between 100 and 400 milliseconds and is expressed in Newtons per second. In fact, it is equivalent to the moment at which we find the steepest slope in the curve. In the image we can see two moments at 100 and 400 milliseconds. If we look at these two moments, we see that there is an RFD of 1500 N/s (150N*0.1s) at 100 milliseconds and an RFD of 1125 N/s (450*0.4s) at 400 milliseconds. We have a similar RFD at 100 as at 400 milliseconds. And what is the RFD for? The RFD is a way of knowing:
- The physical condition of my athlete in the application of force as, for example, in the vertical jump (3).
- The improvement that the athlete has had in the sport gesture.
- The effectiveness of my training program.
If the athlete is able to apply greater force in a shorter execution time, this translates into an improvement in performance.

Source: https://www.abc.es/bienestar/fitness/abci-modela-cuerpo-femenino-entrenamiento-alta-intensidad-201910200410_noticia.html
RFD and the stretch-shortening cycle
Once we know what RFD is, we can deduce that it has a great relationship with the power that a person is able to generate. And as such, power is related to certain physical capacities of our organism and requires a quality musculoskeletal, tendon and ligament system (4). For example, to be able to perform a high-powered sporting gesture, it is necessary that tendons and ligaments are in the best possible shape. When doing a cueing start, the sprinter must exert a great deal of force against the ground, so the ligaments and tendons of the legs will be subjected to a great deal of pressure. In addition, the very stretch-shortening cycle of the muscle puts high demands on the joints.
When an athlete is running multiple RFD moments are produced since each contact with the ground is considered an RFD moment. In the case of sprinters, they endure large forces in minimal times. If my athlete is able to produce more force in the time he is in contact with the ground, as we have advanced at the beginning, whether he is a sprinter or a marathon runner, he will improve his personal best.
However, not in all exercises RFD manifests itself in the same way. A weightlifting athlete performs a stretch-shortening cycle when he is going to lift the barbell but the application time is much longer. In his case, the important moment is when he starts to lift the weight. If our athlete improves his manifestation of strength at the moment of the snatch, his RFD will most likely be better and his lift will be greater.
Why is it good to measure RFD and how is it done?
In team sports one of the determining factors of performance is the speed of movement in the first 2-3 meters and the power of the person. In sports such as indoor football or futsal, basketball, tennis or handball, it is very important to break away from your teammate or perform a movement with the greatest possible explosiveness to achieve the goal or point. This manifestation of strength translates into a faster clearance than your opponent, a more accurate forehand in tennis, or a more agile dribbling in basketball. All these athletes should improve their RFD in the decisive movements of their sport specialty.
Previous research has analyzed the importance of RFD in performance and has seen how weight training helps RFD (5). In the image we can see that before starting weight training the RFD was lower than after.
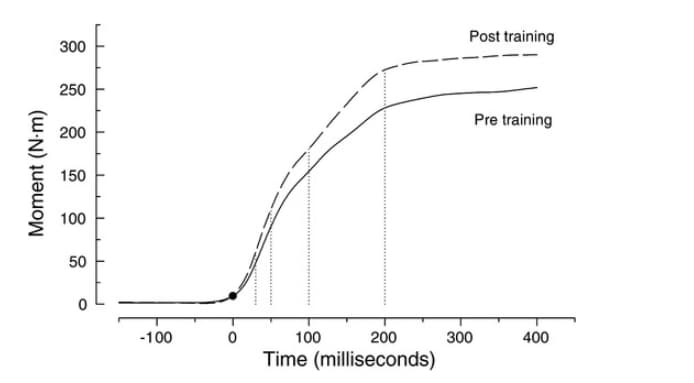
Source: https://pubmed.ncbi.nlm.nih.gov/12235031/
We see that there is a greater slope after training and that the maximum force applied itself is greater. Therefore, what before at 100 milliseconds was a force of approximately 150 Newtons after training at 100 milliseconds a force of approximately 175 Newtons is applied. If we hypothesize this to other sports, it is also observed that muscle strength training can help improve RFD in sprinters, golfers performing the swing or in the pitcher.
When we talk about the way to measure RFD, we have to emphasize that it is not easy. In order to make a good measurement of RFD we must have force platforms that measure the Newtons generated in the sporting gesture. It is important not to confuse RFD with muscle power. Although both variables are related, one measures the force applied per unit of time and the other measures the force applied per unit of velocity. That is, RFD gives a result per unit of time as 400 Newtons in 0.1 seconds, while power gives a result per unit of speed as 200 Newtons at 0.8 meters per second. However, within RFD there are different ways of providing the result and we will emphasize them.
Ways to measure RFD
RFD can be measured in isometric, concentric and eccentric movements. Depending on the sport modality and the sports gesture itself, it can be interesting to monitor the progress of the RFD in a general or specific way. There are different variables within the RFD curve and are:
- Peak RFD
- Time to peak RFD
- Average RFD
- Instantaneous RFD

Source: https://www.iberiansportech.com/shop/forcedecks/
In the following table, we will give an example of the ways to measure RFD. Peak RFD provides information about the moment at which the ratio of force production to application time was greatest, i.e., the moment at which the most force was applied. To do this, we must divide the force expression into similar application times and analyze the applied force in those sections. The time to peak RFD is easy to calculate since if we have the peak RFD, we only have to calculate the time it took from the moment of force application to that peak. The average RFD is the average of each of the sections we have previously mentioned and, finally, the instantaneous RFD refers to the slope of the curve that is traced when we apply force. In this example, we see it clearly:
| Time (ms) | Force (N) | Change of force (N) | Time change (ms) | Relation | RFD (N/s) |
| 0-50 | 100 | 100 | 50 | 100/50 | 2000 |
| 50-100 | 420 | 320 | 50 | 320/50 | 6400 |
| 100-150 | 780 | 360 | 50 | 360/50 | 7200 |
| 150-200 | 1000 | 220 | 50 | 220/50 | 4400 |
Through a very simple spreadsheet we have:
- Peak RFD: 7200 N/s
- Time to peak RFD: 150 milliseconds
- Average RFD: 5000 N/s
- Instantaneous RFD: It would be calculated through the slope of the curve generated by the points we have established.
RFD curve improvements in specific zones
We know that not all sports have the same characteristics and that they require different energy pathways and different application forces. Thus, for example, a javelin thrower does not apply the same force as a sprinter or a gymnast. In fact, if we want to improve the application of force in a specific point of the RFD, we only have to perform a training adapted for them (6).
It has been shown that the principle of specificity is important because if I perform strength work focused on that specific gesture, I will improve the RFD curve at that point. For example, the javelin thrower must apply a very high force with the weight of the javelin. Therefore, he must do work in which the weight does not exceed the weight of the javelin by a large magnitude, but it is a good idea to improve the maximum force at which he moves the load. On the contrary, the sprinter must be able to apply in a very fast way the greatest amount of force so that the slope of the curve formed by his RFD will be greater. Thus, in the following image we can appreciate the change that occurs in the RFD when we follow a training focused on the improvement of maximal strength and when we do it focused on velocity.
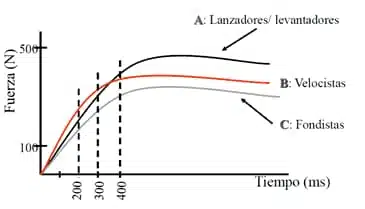
Source: https://mundoentrenamiento.com/fuerza-en-el-deporte/
Conclusion
Finally, let’s emphasize that RFD is a way to monitor the performance of our athlete. It is important to note that the RFD depends on the type of training that we carry out and that it is a way to quantify the expression of force based on time. The higher the RFD value (8000 N/s) the greater the expression of strength and as we have started commenting in this post, strength is the physical capacity from which all others derive.
To conclude, the recommendation we give you from the Vitruve blog is that monitoring the evolution of the RFD can be a key parameter in the performance of your athlete. The RFD helps us to know how the athlete is applying strength and if he is improving with our training. Do not hesitate to give a plus to your training and your planning by measuring the RFD.
To conclude, the recommendation we give you from Vitruve’s blog is that monitoring the evolution of RFD can be a key parameter in the performance of your athlete. The RFD helps us to know how the athlete is applying the force and if it is improving with our training. Do not hesitate to give a plus to your training and planning by measuring RFD.
Unai Adrián Perez de Arrilucea Le Floc’h
References
- Bo K, Aschehoug A. Strength training. Evidence-based physical therapy for the pelvic floor: bridging science and clinical practice Edinburgh: Churchill Livingstone. 2007:119-32.
- Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J. Rate of force development: physiological and methodological considerations. European journal of applied physiology. 2016;116:1091-116.
- McLellan CP, Lovell DI, Gass GC. The role of rate of force development on vertical jump performance. The Journal of Strength & Conditioning Research. 2011;25(2):379-85.
- Taber C, Bellon C, Abbott H, Bingham GE. Roles of maximal strength and rate of force development in maximizing muscular power. Strength & Conditioning Journal. 2016;38(1):71-8.
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. Journal of applied physiology. 2002;93(4):1318-26.
- Holtermann A, Roeleveld K, Vereijken B, Ettema G. The effect of rate of force development on maximal force production: acute and training-related aspects. European journal of applied physiology. 2007;99:605-13.

