30 de December de 2020
Post-Activation Potentiation: underlying mechanisms, applications, and constraints.
Exercise can be seen as a type of stressor which, like any other stressor, is able to exert a biological response (or stress response). A common feature of all stressors is that there is an acute phase, during which homeostatic adjustments occur; a more chronic phase, during which the stressor can be accommodated by the adaptations; and finally a phase of exhaustion, during which maladaptations occur.
Exercise scientist, have been historically obsessed with the chronic response to the homeostatic perturbation mediated by exercise (aka adaptation) such as hypertrophy. However, muscle contraction is able to elicit a vast variety of acute homeostatic responses such as altered blood flow to the active muscles; increased heart rate; increased breathing rate; increased oxygen consumption; increased rate of sweating; increased body temperature; secretion of stress hormones such as adrenocorticotropic hormone (ACTH), cortisol, and catecholamines; increased glycolytic flux; and altered recruitment of muscles. These changes are transient and return to baseline levels after exercise1.
We, as strength and conditioning professionals or coaches, are constantly searching and trying to implement a variety of training methods to improve our athlete’s performance nos only chronically but also acutely. Every time we find ourselves struggling to come up with a state of the art warm-up protocol to boost a training session or match day performance, deep inside we are just trying to manipulate every single physiological process with power-enhancing characteristics to our advantage.
A strength training technique that has become the subject of recent investigation and interest by coaches and athletes is post-activation potentiation (PAP), a phenomenon by which muscle function is increased as a direct result of its contractile history2. PAP is the physiological process upon which complex and contrast training is based; both methodologies were developed to induce a muscular state of “preparedness” or potentiation.
PAP has been defined as an enhanced muscle contractile response for a given level of stimulation following an intense voluntary contraction, which is measured as the maximum twitch force evoked by supramaximal electrical stimulation3. However, many forms of potentiation have been described in the literature usually and mistakenly referred to as PAP. Chances are you are not actually measuring or inferring PAP (which involve confirmation by twitch stimulations) but post-activation potentiation performance enhancements (PAPE) which is basically the voluntary force or power enhancement seen after a high-intensity exercise-based warm-up attributed to other factors that impact muscle function (e.g., muscle temperature, activation level/learning)4.
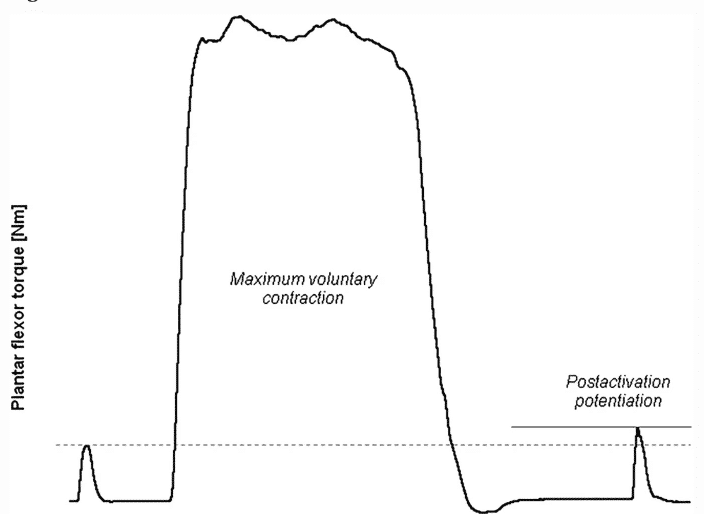
Figure 1 Example of the measurement of post-activation potentiation (PAP) in the plantar flexor muscles. A baseline twitch is artificially evoked in the resting plantar flexors. Two seconds following a conditioning maximum voluntary contraction, the evoked twitch has a greater peak torque compared with the baseline twitch. The increment from baseline twitch peak torque (dashed line) to post-contraction twitch peak torque (solid line) corresponds to the extent of PAP5
Brief History and evolution of the terminology
The first works on the potentiating capacity of certain muscular regimes date from the beginnings of exercise physiology in the 19th century. The oldest term used in the literature appears to be “treppe” (staircase) in the paper “The cause of the treppe”6 in 1906 referring to the repeated responses of a given tissue to repeated and equal stimuli increase for a time in intensity. Although, the concept was not new at the time, originated from the innovative work done by germans researchers in 1865 and 18717.
Since then, three different forms of potentiation have been described in the literature: staircase, post-tetanic potentiation, and PAP4. All of which have a common characteristic; enhancement of the contractile response after the application of a conditioning stimulus. Moreover, the primary difference between the three forms of potentiation is the conditioning stimulus; in staircase, the conditioning contractions comprise repeated, low-frequency electrical stimulations where contractions sequentially increase in amplitude; in post-tetanic potentiation, a brief high-frequency train of electrical stimulation acts as the conditioning contraction 8,9. ; in PAP, the enhanced contractile response (muscle twitch) is evoked by voluntary muscle activation4,10.
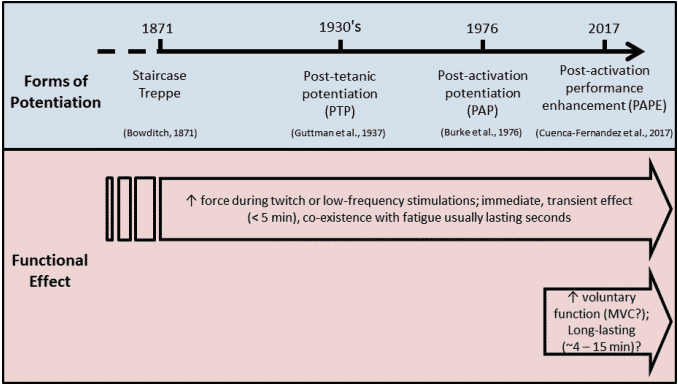
Figure 2 Many forms of potentiation have been described in the literature since 19th century4
During the last 50 years, researchers have been studying PAP in different kinds of subjects, conditions, and methodologies making great progress toward a better understanding of its mechanisms, implications, and best practices. However, given that for a proper PAP assessment it´s mandatory to incorporate an electrically evoked twitch or low-frequency tetanic stimulation in order to determine its magnitude at the point of voluntary muscle testing as well as proper management of the time course of the PAP effect, it is not always clear whether “classic” PAP underpins the performance enhancement4. Furthermore, it has been hypothesized that underpinning these performance enhancements in vivo with a different time course may be due to a different mechanism. Thus, it has been proposed that the term “post-activation performance enhancement” (PAPE) should be used when high-intensity voluntary conditioning contraction(s) are performed with the intent of enhancing subsequent voluntary, rather than electrically evoked (twitch), force production4
Mechanism
Before starting to talk about the physiological mechanism responsible for the physiological phenomenon of PAP, it´s important to describe the muscle contraction at the molecular level.
For muscle contraction to occur, the electrical potential, generated in the motor neuron, has to penetrate to the contractile machinery in the muscle, this is achieved by transmitting potentials along with the system of transverse tubules (T tubules). The voltage-sensitive dihydropyridine receptors located in the T tubules are activated by the depolarization of the membrane induced by the action potential and thus interact directly with the ryanodine receptor (Ca2 + releasing channel) located in the sarcoplasmic reticulum. Subsequently, the depolarization of the membrane induces the release of the Ca2 + contained in the sarcoplasmic reticulum into the space adjacent to the myofibrils and activates the contractile mechanisms11,12.
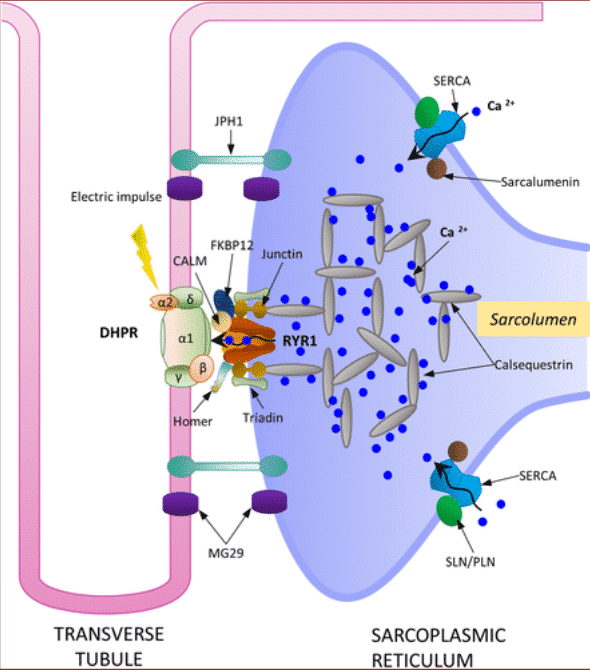
Figure 3 T Tubule structure and function.11
When the sarcoplasmic reticulum is depolarized, the Ca2 + that it contains in its terminal cisterns is discharged into the cytoplasm and binds with Troponin C (TpC), this binding causes the link between troponin and actin to be weakened, allowing tropomyosin to move laterally and expose the active site where actin binds to myosin. For every Ca2 + that binds to troponin, 7 binding sites for myosin are uncovered. It is now that the myosin heads are attached to the actin-binding sites and once attached, the myosin heads act as hinges moving and dragging the actin chain.
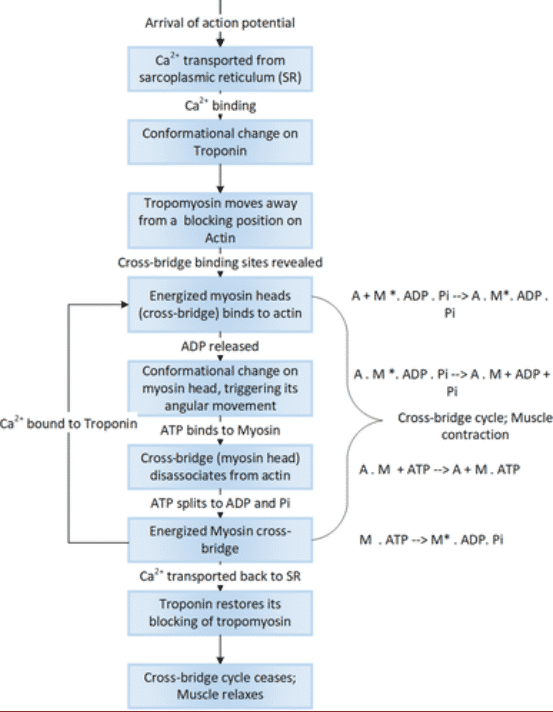
Figure 4 Muscle Contraction Process 11
Muscle contraction consists essentially of the union and separation of the S-1 head of myosin with the F-actin filaments. The binding of actin to myosin generates conformational changes dependent on ADP or ATP, among which the S-1 head stands out. These changes generate the so-called power stroke, which directs the movement of the actin filaments through the myosin filaments. The energy of this process is supplied by ATP, which is hydrolyzed to ADP and Pi; however, the power stroke occurs as a result of conformational changes when ADP detaches from the myosin heads11.
Muscle Contraction
3.1. Molecular
Myosin is a hexameric protein that contributes 55% to the weight of muscle protein and is composed of a fibrous end of two interspersed helices, each helix has a globular head attached to each end. The hexamer consists of a pair of heavy chains and two different light chains (essential and regulatory; ELC and RLC). Each RLC has a specific binding site for the incorporation of phosphate groups. RLC phosphorylation is catalyzed by the light chain myosin kinase enzyme, which is activated when Ca2 + molecules are released from the sarcoplasmic reticulum during muscle contraction12–15.
Once calcium reaches the cytoplasm of the muscle cell, binds to a protein called calmodulin, forming a calcium-calmodulin complex16. Calcium-calmodulin complex activates an enzyme called myosin light chain kinase. This enzyme catalyzes the incorporation of phosphates groups to the myosin light chains, phosphorylating them, and altering the state of the cross-bridges according to the state of phosphorylation13,15.
There is abundant scientific evidence showing that the physiological phenomenon of potentiation results from the phosphorylation of RLCs 17–19. Numerous studies have shown the existence of a significant correlation between RLCs phosphorylation and the magnitude of the potentiation4.
Phosphorylation of RLCs is thought to enhance subsequent contractions by altering the structure of the myosin head20. The phosphorylation of RLCs causes the myosin heads to move away from the backbone of the thick filament, decreasing the distance between the thin and thick filaments, thus increasing the speed at which it can approach actin. It has also been shown that RLC phosphorylation makes the actin-myosin interaction more sensitive to myoplasmic Ca2 +20,21. Furthermore, the RLCs phosphorylation would result in a greater amount of active cross-bridge conformation. This could partly explain the increase in both force production and the rate of force development reported in mammalian skeletal muscle after conditioning stimulus22,23.
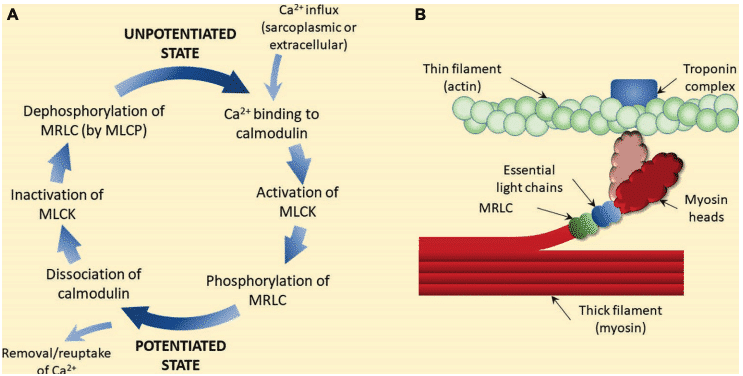
Figure 5 Proposed mechanism of post-activation potentiation (PAP)4
On the other hand, the evidence that RLCs phosphorylation is the main mechanism behind PAP does not exclude the participation of other mechanisms. Changes in muscle architecture; specifically related to the pennation angle (AP), have been shown to be a potential responsible for the generation of PAP. The AP is made up of fascicles and internal aponeurosis, which reflects the orientation of the muscle fibers in relation to the connective tissue. For this reason, any change in it will affect the transmission of force from the muscles to the tendons and bones24,25.
3.2. Neural
Other authors, suggested that the physiological basis for potentiation is based on a neural effect. Verkhoshansky proposed that any stimulus prior to motor action, whether momentary or not, generates a “trace in the nervous system” that allows maintaining the levels of force obtained for a certain amount of time and even improve them which latter was the theoretical background behind the shock method developed by the aforementioned author. This enhancement could be achieved due to an increased level of voluntary neural drive.
Neural factors such as increased recruitment of high-threshold motor units, increased activity of the synergistic muscles, increased reflex activity, and inhibition of the Golgi apparatus could directly or indirectly contribute to the generation of PAP. The increase in neural activity generates greater recruitment of high-threshold motor units, improves the synchronization between them, reduces presynaptic inhibition, and generates an increase in impulses from the central nervous system (CNS) towards the periphery. The triggering stimulus (pre-contraction) increases the probability of initiating an action potential whose generated excitation can last several minutes, increasing the postsynaptic potentials that lead to a greater generation of force.
However, increases in EMG are usually not observed, even when improvements in functional performance are clearly detected26–28 and despite indications that neural changes may be triggered by voluntary activity, there is a lack of evidence in support of the theory that increases in neural drive to the muscle contribute to increases in complex voluntary muscular performance seen in many studies4
PAP vs PAPE
High-intensity muscle contractions such as those used in maximal exercise or heavy lifting require maximal or near-maximal levels of muscle activation, and thus PAP cannot directly influence them. However, it has been shown that PAP can shift the relationship between force and Ca+ concentration therefore it can increase the force output at a given Ca+ release (more force with less activation, which can be seen as an increase in the RFD) especially during brief contractions where maximal force cannot be reached22,29–31. This may thus influence performance during intensive, short-duration activities such as jumping or kicking32.
The evidence for the existence of PAP with respect to contractile properties is abundant. However, the evidence for PAP in performance-related tasks has been somewhat confusing due to studies that have failed to observe improvements or, if observed, it cannot be attributed to PAP. While some residual PAP might influence PAPE in its earliest stages after a conditioning activity, other mechanisms must play a significant role in PAPE.
Proof that PAP has underpinned performance enhancement (i.e., PAPE) can only be provided when the outcome variable measured during a voluntary contraction changes with the same time course as the classic PAP response, which is commonly estimated through measurement of the peak muscle twitch force4. Unfortunately, these types of study designs are not abundant.
As we already pointed out, It seems that PAP is different from PAPE and the term PAPE should be used in preference in order to identify the possibility that a separate phenomenon is taking place than classical PAP33. However, one key aspect of PAPE that requires a better understanding is the lack of a performance enhancement effect during time points where PAP is manifesting (3-4 min) since PAP should be causing PAPE.
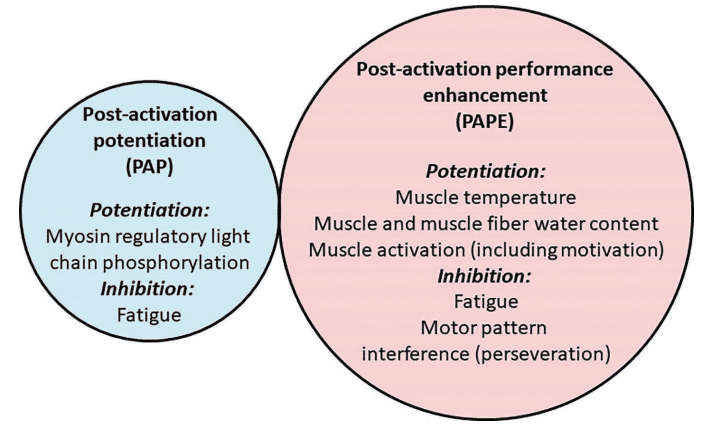 Figure 6 Potential factors influencing post-activation potentiation (PAP) and post-activation performance enhancement (PAPE)4
Figure 6 Potential factors influencing post-activation potentiation (PAP) and post-activation performance enhancement (PAPE)4
There is still no scientific consensus. However, the dominant theory behind this lack of effect is a coexistence of potentiation and fatigue25,34,35. Different types of contractions would generate potentiating effects to a greater or lesser extent, and this could be related to the level of neuromuscular fatigue they produce. After a maximal or near-maximal muscle contraction, the muscles are in a fatigued and potentiated state simultaneously. The enhanced state remains for a given period of time, after which fatigue subsides and provides a “window” during which the athlete may be able to obtain the ergogenic benefit of the potentiated state, although it seems that it is not possible to generalize recovery durations36,37. Furthermore, recovery duration shows a large inter-individual variability that is associated with numerous factors such as strength level, training experience, and myotypology36–38.
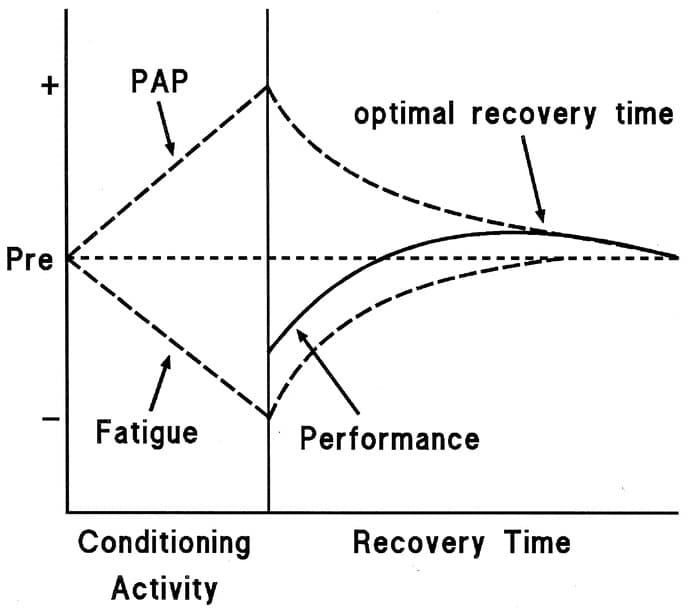
Figure 7 Coexistance of PAP and Fatigue 32
Another possibility is that the lack of PAPE in the minutes after a conditioning activity, despite PAP being significant, results from a motor pattern interference effect (often called “perseveration” since the motor pattern of one task perseveres while a similar, subsequent task is commenced). However, it is also likely that other physiological changes augment voluntary muscle function at the time points where PAPE has been observed, such as increases in muscle temperature or coordination (learning or motivational effects), or improvements in muscle function through non-RLCs mechanisms such as intracellular water accumulation or changes in blood flow4.
Methods taking advantage of PAP, such as complex or contrast, are likely useful to enhance mechanical power and therefore the performance and/or the training stimulus of an explosive sports activity. The evidence on the practical application of the PAP on explosive activities is still inconclusive given the aforementioned difference between PAP and PAPE. It seems that PAPE response could just be the outcome of a good, comprehensive, and successful warm-up.
Bibliography
1.Lambert, M. I. General Adaptations to Exercise: Acute Versus Chronic and Strength Versus Endurance Training. in Exercise and Human Reproduction 93–100 (Springer New York, 2016). doi:10.1007/978-1-4939-3402-7_6.
2.Robbins, D. W. & Docherty, D. Effect of Loading on Enhancement of Power Performance Over Three Consecutive Trials. The Journal of Strength and Conditioning Research 19, 898 (2005).
3.MacIntosh, B. R., Robillard, M.-E. & Tomaras, E. K. Should postactivation potentiation be the goal of your warm-up? Applied Physiology, Nutrition, and Metabolism 37, 546–550 (2012).
4.Blazevich, A. J. & Babault, N. Post-activation Potentiation Versus Post-activation Performance Enhancement in Humans: Historical Perspective, Underlying Mechanisms, and Current Issues. Frontiers in Physiology 10, (2019).
5.Prieske, O., Behrens, M., Chaabene, H., Granacher, U. & Maffiuletti, N. A. Time to Differentiate Postactivation ‘Potentiation’ from ‘Performance Enhancement’ in the Strength and Conditioning Community. Sports Medicine 50, 1559–1565 (2020).
6.Lee, F. S. The cause of the treppe. Experimental Biology and Medicine 4, 22–23 (1906).
7.Bowditch, H. l] ber die Eigenth~ imlichkeiten der Reizbarkeit, welche die Muskelfasern des Herzens zeigen. Berichte Ober die Verhandlungen der k6nighch s~ chsischen Gesellschaft der Wissenschaften zu Leipzig. Mathemat.-Phys. Classe 23, 652–589.
8.Guttman, S. A., Horton, R. G. & Wilber, D. T. Enhancement of Muscle Contraction after Tetanus. Experimental Biology and Medicine 34, 219–221 (1936).
9.Brown, G. L. & von Euler, U. S. The after effects of a tetanus on mammalian muscle. The Journal of Physiology 93, 39–60 (1938).
10.Burke, R. E., Rudomin, P. & Zajac, F. E., III. The effect of activation history on tension production by individual muscle units. Brain Research 109, 515–529 (1976).
11.Mukund, K. & Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdisciplinary Reviews: Systems Biology and Medicine (2019) doi:10.1002/wsbm.1462.
12.Craig, R. & Woodhead, J. L. Structure and function of myosin filaments. Current Opinion in Structural Biology 16, 204–212 (2006).
13.Szczesna, D. et al. Phosphorylation of the regulatory light chains of myosin affects Ca2+sensitivity of skeletal muscle contraction. Journal of Applied Physiology 92, 1661–1670 (2002).
14.Manning, D. R. & Stull, J. T. Myosin light chain phosphorylation-dephosphorylation in mammalian skeletal muscle. American Journal of Physiology-Cell Physiology 242, C234–C241 (1982).
15.Davis, J. S., Satorius, C. L. & Epstein, N. D. Kinetic Effects of Myosin Regulatory Light Chain Phosphorylation on Skeletal Muscle Contraction. Biophysical Journal 83, 359–370 (2002).
16.Moore, R. L. & Stull, J. T. Myosin light chain phosphorylation in fast and slow skeletal muscles in situ. American Journal of Physiology-Cell Physiology 247, C462–C471 (1984).
17.Grange, R. W., Cory, C. R., Vandenboom, R. & Houston, M. E. Myosin phosphorylation augments force-displacement and force-velocity relationships of mouse fast muscle. American Journal of Physiology-Cell Physiology 269, C713–C724 (1995).
18.Houston, M. E., Lingley, M. D., Stuart, D. S. & Grange, R. W. Myosin light chain phosphorylation in intact human muscle. FEBS Letters 219, 469–471 (1987).
19.Sweeney, H. L., Bowman, B. F. & Stull, J. T. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. American Journal of Physiology-Cell Physiology 264, C1085–C1095 (1993).
20.Szczesna-Cordary, D. Regulatory Light Chains of Striated Muscle Myosin. Structure, Function and Malfunction. Current Drug Target -Cardiovascular & Hematological Disorders 3, 187–197 (2003).
21.Baudry, S., Klass, M. & Duchateau, J. Postactivation potentiation of short tetanic contractions is differently influenced by stimulation frequency in young and elderly adults. European Journal of Applied Physiology 103, 449–459 (2008).
22.Vandenboom, R., Xeni, J., Bestic, N. M. & Houston, M. E. Increased force development rates of fatigued mouse skeletal muscle are graded to myosin light chain phosphate content. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 272, R1980–R1984 (1997).
23.Vandenboom, R., Grange, R. W. & Houston, M. E. Myosin phosphorylation enhances rate of force development in fast-twitch skeletal muscle. American Journal of Physiology-Cell Physiology 268, C596–C603 (1995).
24.Fukunaga, T., Ichinose, Y., Ito, M., Kawakami, Y. & Fukashiro, S. Determination of fascicle length and pennation in a contracting human muscle in vivo. Journal of Applied Physiology 82, 354–358 (1997).
25.Hodgson, M., Docherty, D. & Robbins, D. Post-Activation Potentiation. Sports Medicine 35, 585–595 (2005).
26.Hough, P. A., Ross, E. Z. & Howatson, G. Effects of Dynamic and Static Stretching on Vertical Jump Performance and Electromyographic Activity. Journal of Strength and Conditioning Research 23, 507–512 (2009).
27.Seitz, L. B., Trajano, G. S., Dal Maso, F., Haff, G. G. & Blazevich, A. J. Postactivation potentiation during voluntary contractions after continued knee extensor task-specific practice. Applied Physiology, Nutrition, and Metabolism 40, 230–237 (2015).
28.Mina, M. A., Blazevich, A. J., Giakas, G., Seitz, L. B. & Kay, A. D. Chain-loaded variable resistance warm-up improves free-weight maximal back squat performance. European Journal of Sport Science 16, 932–939 (2016).
29.Tillin, N. A. & Bishop, D. Factors Modulating Post-Activation Potentiation and its Effect on Performance of Subsequent Explosive Activities. Sports Medicine 39, 147–166 (2009).
30.Vandenboom, R., Gittings, W., Smith, I. C., Grange, R. W. & Stull, J. T. Myosin phosphorylation and force potentiation in skeletal muscle: evidence from animal models. Journal of Muscle Research and Cell Motility 34, 317–332 (2013).
31.Vandenboom, R., Grange, R. W. & Houston, M. E. Threshold for force potentiation associated with skeletal myosin phosphorylation. American Journal of Physiology-Cell Physiology 265, C1456–C1462 (1993).
32.Sale, D. G. Postactivation Potentiation: Role in Human Performance. Exercise and Sport Sciences Reviews 30, 138–143 (2002).
33.Cuenca-Fernández, F. et al. Nonlocalized postactivation performance enhancement (PAPE) effects in trained athletes: a pilot study. Applied Physiology, Nutrition, and Metabolism 42, 1122–1125 (2017).
34.Fowles, J. R. & Green, H. J. Coexistence of potentiation and low-frequency fatigue during voluntary exercise in human skeletal muscle. Canadian Journal of Physiology and Pharmacology 81, 1092–1100 (2003).
35.Rassier, D. E. & MacIntosh, B. R. Coexistence of potentiation and fatigue in skeletal muscle. Brazilian Journal of Medical and Biological Research 33, 499–508 (2000).
36.Gołaś, A., Maszczyk, A., Zajac, A., Mikołajec, K. & Stastny, P. Optimizing post activation potentiation for explosive activities in competitive sports. Journal of Human Kinetics 52, 95–106 (2016).
37.Naclerio, F. et al. Effects of Three Different Conditioning Activity Volumes on the Optimal Recovery Time for Potentiation in College Athletes. Journal of Strength and Conditioning Research 29, 2579–2585 (2015).
38.Chen, Z.-R., Lo, S.-L., Wang, M.-H., Yu, C.-F. & Peng, H.-T. Can Different Complex Training Improve the Individual Phenomenon of Post-Activation Potentiation? Journal of Human Kinetics 56, 167–175 (2017).

